MRNA vaccines — a new era in vaccinology
Mucosal vaccines — fortifying the frontiers
Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection. In principle, the induction of adaptive immunity at mucosal sites, involving secretory antibody responses and tissue-resident T cells, has the capacity to prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms. Although numerous effective mucosal vaccines are in use, the major advances seen with injectable vaccines (including adjuvanted subunit antigens, RNA and DNA vaccines) have not yet been translated into licensed mucosal vaccines, which currently comprise solely live attenuated and inactivated whole-cell preparations. The identification of safe and effective mucosal adjuvants allied to innovative antigen discovery and delivery strategies is key to advancing mucosal vaccines. Significant progress has been made in resolving the mechanisms that regulate innate and adaptive mucosal immunity and in understanding the crosstalk between mucosal sites, and this provides valuable pointers to inform mucosal adjuvant design. In particular, increased knowledge on mucosal antigen-presenting cells, innate lymphoid cell populations and resident memory cells at mucosal sites highlights attractive targets for vaccine design. Exploiting these insights will allow new vaccine technologies to be leveraged to facilitate rational mucosal vaccine design for pathogens including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and for cancer.
Introduction
The global burden of mortality and morbidity associated with infectious diseases caused by mucosal pathogens remains unacceptably high. Indeed, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic provides a brutal reminder of the continual threat of novel mucosal infectious challenges, in addition to the threat posed by many widespread mucosal infections for which no or only suboptimal vaccines exist. Now more than ever, there is a clear focus on vaccine requirements for respiratory pathogens but, importantly, new and improved vaccines are also urgently needed for numerous enteric pathogens and other agents such as those causing sexually transmitted diseases (STDs) and oncogenic viruses that gain access through the mucosae.
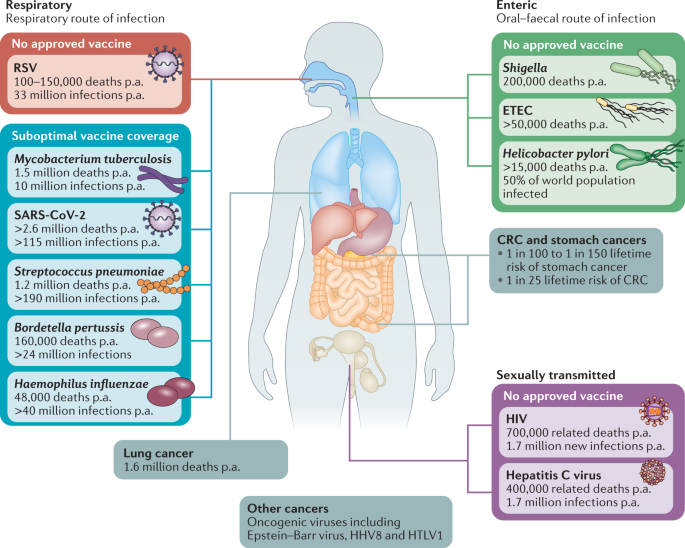
Respiratory, enteric and sexually transmitted infections constitute prominent causes of death worldwide, and this is exacerbated in low-income regions. Aetiological agents shown are vaccine targetable, but there remains an unmet need for new or improved vaccination approaches to address global vaccine coverage. Mucosal vaccination strategies hold promise to address this unmet need, providing more robust mucosal immunity and an alternative to parenteral vaccination. In addition to their centrality in the pathogenesis of infectious disease, mucosal tissues are frequent sites of tumour development and mucosal vaccination strategies may play a role in the prophylactic and therapeutic targeting of these malignancies. CRC, colorectal cancer; ETEC, enterotoxigenic Escherichia coli; p.a., per annum; RSV, respiratory syncytial disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Enteric pathogens causing diarrhoeal disease are the eighth leading cause of death worldwide, with children, in particular, at risk 14 . Among these, Shigella and enterotoxigenic Escherichia coli (ETEC) have an urgent vaccine requirement (Fig. 1). Enteric pathogens and associated acute and chronic infections have a stark impact on the livelihoods of at-risk individuals in lower-income countries. Aside from diarrhoeal disease, the impact of such infections on physical and cognitive development is becoming more apparent 14 , not only highlighting the need for vaccine development but also impacting how we determine vaccine efficacy. Lack of moderate-to-severe symptoms may not be an adequate correlate of protection — prevention of colonization and/or low-grade infection may be the crucial determinant. The World Health Organization (WHO) has endeavoured to end cholera by 2030 through implementation of widespread preventive measures, including vaccination 15 , providing a challenge to oral cholera vaccine manufacturers globally. This may be addressed through successful development of lower-cost alternative oral cholera vaccines such as Hillchol, which is currently under clinical evaluation 16 .
Mucosal vaccines targeting the genital tract have the potential to combat STDs and local tumours, which is important given that cervical cancer represents the fourth most common cancer in women 17 . Clearly, there is an enormous need for an effective HIV vaccine and given the intestinal tropism of the virus 18 , mucosal vaccine strategies are warranted 19 . Additionally, the emergence of multidrug-resistant STDs is of concern and could be combatted through preventive mucosal vaccine strategies.
Whereas most licensed vaccines are currently administered by injection, mucosal vaccines can outperform parenteral vaccination strategies in eliciting protective mucosal immune responses that block infection or transmission 20,21 . The nature of the infection must be considered from the outset in designing mucosal vaccines or, indeed, in assessing whether targeting the mucosal route is necessary. For example, in the case of enteric pathogens, the infections may be invasive (as is seen in typhoid and polio), partially/locally invasive (as seen in shigellosis) or strictly mucosal (as seen for cholera and ETEC infections) 22,23 . This will impact on whether a systemic immune response is an appropriate objective, or whether predominantly mucosal or both mucosal and systemic immunity would be more effective. In this context, the nature of the mucosal surface (for example, the uninflamed small intestine versus the lower respiratory tract) will influence the accessibility of circulating antibodies, the nature of the dominant antibody isotype and the transport mechanism governing access of antibodies to mucosal infectious pathogens 24,25 .
Strong mucosal cellular and humoral immune responses have the potential to induce sterilizing immunity by impeding pathogen binding to and uptake across epithelial surfaces. However, there are significant hurdles to mucosal vaccine development, including incomplete knowledge of the nature of protective mucosal immune responses. Advancing new mucosal vaccines and improving existing vaccines requires innovative adjuvant approaches and delivery strategies, which is the focus of this Review. Given the specific architecture of the mucosal surfaces and their unique cellular composition, vaccine strategies should be specifically tailored for the target site rather than redeploying effective injectable vaccines. In any case, many adjuvants that are effective by injection are not optimal for mucosal delivery.
Lessons from licensed mucosal vaccines
Over recent decades, there has been a broad shift from injectable whole-cell killed and attenuated vaccines towards adjuvanted subunit and, more recently, viral vectored, RNA and DNA vaccines 26,27 . This can reduce the potential for excessive reactogenicity and is facilitated by advances in antigen discovery, adjuvants and delivery systems. However, the landscape for mucosal vaccines is very different. Of the nine mucosal vaccines approved for use in humans — eight oral and one intranasal — all are either live attenuated or whole-cell inactivated vaccine formulations (Fig. 2). This current dichotomy in approaches is, in part, due to greater tolerability of orally administered whole-cell killed antigens, the susceptibility of unprotected subunit antigens to degradation and clearance, and, crucially, a lack of proven mucosal adjuvants.
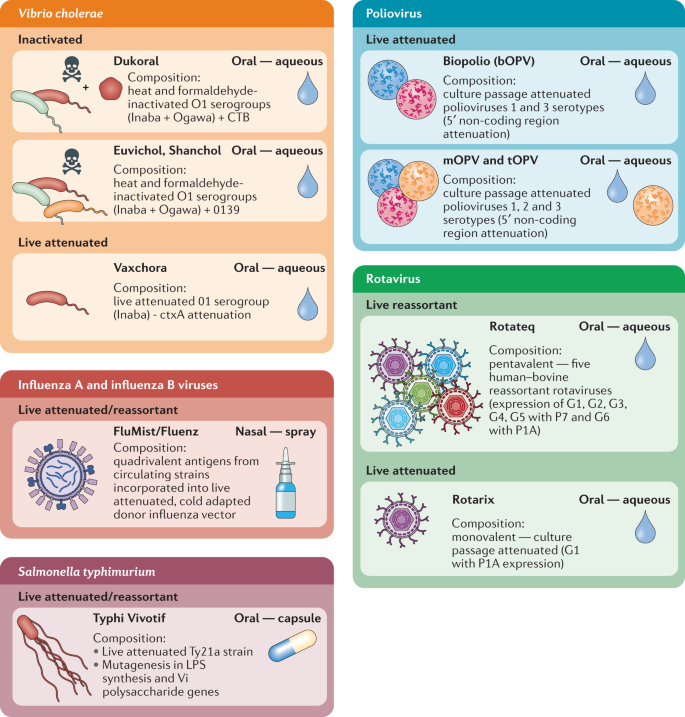
Eight oral vaccines are currently licensed for use against cholera, salmonella, poliovirus and rotavirus. Live attenuated influenza vaccines remain the sole licenced intranasal vaccines. To date, live attenuated and inactivated vaccines have proved the most successful platforms for mucosal vaccine design. CTB, cholera toxin B subunit; LPS, lipopolysaccharide.
Currently, the only subunit antigen included in a licensed mucosal vaccine is cholera toxin B subunit (CTB), included as an additional component of the killed whole-cell Vibrio cholerae vaccine Dukoral. CTB cannot be regarded as a ‘model’ subunit antigen as this is the binding component of cholera toxin — it binds with high affinity to GM1 on epithelial cells and is highly immunogenic 28,29,30 (Box 1). Nevertheless, this does indicate that potent immune responses can be induced against an orally administered purified protein even if this is in the presence of whole bacteria. The tolerability of oral whole-cell antigens is instructive as, although adjuvanted subunits are preferable for parenteral formulations, leveraging potent mucosal adjuvants with whole-cell antigens may be a more productive approach for mucosal vaccination, especially via oral routes.
Developing whole-cell antigens as a platform offers potential for combination with purified subunits but also, perhaps more encouragingly, for overexpression of antigens on whole cells — ETVAX provides a stellar example of this 31 . Developed at the University of Gothenburg in collaboration with Scandinavian Biopharma, ETVAX comprises four E. coli strains engineered to overexpress colonization factor antigens on the bacterial surface, namely CFA/I, CS3, CS5 and CS6, in combination with LCTBA 31,32 (a CTB and E. coli heat-labile enterotoxin B subunit (LTB) hybrid; see Box 1). Overexpression of antigens on inactivated whole bacteria is thus a promising approach to increase immunogenicity, leveraging the adjuvanticity of inactivated bacteria while helping to minimize the vaccine dose required. This approach may also be applied to inactivated viruses or virus-like particles, taking advantage of their inherent immunogenicity, particulate antigen presentation and well-established expression systems 33,34 .
Aside from Dukoral, Euvichol and Shanchol, the other licensed mucosal vaccines are all live attenuated bacteria (Salmonella enterica subsp. enterica serovar Typhimurium) or attenuated and/or reassortant viruses delivered orally (polio vaccine, rotavirus) or nasally (influenza A and influenza B viruses). Overall, this highlights the tolerability and effectiveness of mucosal attenuated and whole-cell vaccines but also points to the key question of why the successful shift to more modern vaccine strategies has not yet occurred for mucosal vaccines.
Box 1 CTB — mucosal subunit antigen or adjuvant?
The pentameric cholera toxin B subunit (CTB) has been successfully and safely incorporated in recombinant form into the oral cholera vaccine Dukoral since 1991. It represents the only subunit antigen incorporated in a licensed mucosal vaccine to date. CTB binds to the membrane ganglioside GM1 (ref. 143 ) and fucosylated glycans 144,145 on cells including enterocytes, microfold (M) cells, macrophages and dendritic cells. As a result, CTB has the potential to promote effective delivery of bound antigens to mucosal antigen-presenting cells (APCs) 146 . Although CTB has in the past been classed as an adjuvant, this definition was complicated by the presence of residual cholera toxin or lipopolysaccharide (LPS) in CTB preparations. Indeed, CTB is now well established as an effective mediator of tolerance to attached or admixed antigens by oral and intranasal routes 147,148,149 . It was also proposed that orally delivered CTB can promote intestinal repair and healing responses 150 . In human studies, CTB has been shown to also promote induction of antigen-specific local IgA and systemic IgG responses when administered via rectal and intranasal routes 151,152 . Its inclusion in Dukoral is primarily to induce intestinal and circulating cholera toxin-specific antibodies, which can contribute to short-term protection against cholera 153 and cross-protection against enterotoxigenic Escherichia coli (ETEC) via shared epitopes in E. coli heat-labile enterotoxin B subunit (LTB) 154,155 . Similarly, LCTBA (a CTB and LTB hybrid) is included in the candidate ETEC vaccine ETVAX, expanding the number of antigenic targets and cross-protection 31,32 . Recently, CTB has been shown to promote activation and expansion of polyfunctional T helper 1 (TH1) cells and TH17 cells when given intradermally alongside a DEC205 + dendritic cell-targeted antigen; notably, this included induction of local and intestinal protective tissue-resident memory T (TRM) cells 29 . This highlights the potential for incorporation of CTB in parenteral–mucosal push–pull vaccination strategies.
Vaccine lessons from mucosal tissues
Unique aspects of mucosal immune responses
There are many distinctive features of mucosal immune responses that impact on mucosal vaccine design, ranging from the structure and location of immune inductive sites to the type of effector and memory cells induced and their longevity and location. The mucosal immune system can be broadly categorized into inductive sites where antigen-specific B cell and T cell responses are initiated and effector sites (such as the lamina propria and epithelium) where the adaptive immune responses are mediated. The nature of the inductive sites varies between species and also between different mucosae. In the case of the intestine, the inductive sites are the gut-associated lymphoid tissue and the intestine-draining mesenteric lymph nodes. Gut-associated lymphoid tissue in humans and mice comprises Peyer’s patches and isolated lymphoid follicles 35 . The connection between inductive and effector sites is facilitated by selective expression of integrins and chemokine receptors on B cells 36 and T cells 37 . For example, in the case of the small intestine, imprinting of α4β7 integrin and CC-chemokine receptor 9 (CCR9) expression on lymphocytes is key for tissue-specific homing of cells. Although mucosal immune responses are compartmentalized, there is crosstalk between different mucosae, and it is thus possible to vaccinate at a single mucosal site but also promote immune responses at distant mucosal sites 38 . Understanding the nature of the signals regulating such homing in a human context is critical to allow design of novel mucosal vaccines that can potentially target mucosae distant from the site of vaccination.
Mucosal sites cover a surface area of 30–40 m 2 in humans 39 predominantly in the gastrointestinal tract, respiratory tract, urogenital tract and ocular cavities, playing a crucial role in homeostasis and interactions with the microbiome, dietary antigens and other environmental material. As a result, they constitute prominent points of pathogen entry and are often sites of tumour development. This high antigenic load and constant exposure to microbes necessitates mucosal immunoregulatory mechanisms that are vital to maintain homeostasis and prevent damaging chronic inflammatory responses 40 . This has significant implications with regard to vaccine development, for example, many Toll-like receptor (TLR) agonists that are effective adjuvants when injected parenterally have poor efficacy when administered orally. This results, at least in part, from tolerization of intestinal antigen-presenting cells (APCs) to pathogen-associated molecular patterns, particularly TLR ligands to which they are continuously exposed via the microbiome 41 , and also as a result of the basolateral rather than luminal expression of TLRs at epithelial surfaces. Detailed knowledge on mucosal responsiveness to pathogen-associated molecular patterns, responsive target cells and their location is critical so that productive target pathways can be identified for adjuvant discovery and optimization.
A recent report demonstrated that proximal intestinal gut-draining lymph nodes preferentially supported regulatory T cell responses whereas distal gut-draining lymph nodes supported the induction of effector T helper cells 42 . These insights into the balance of regulatory and effector responses can inform vaccine design — if antigen uptake in proximal regions of the small intestine preferentially enhances tolerogenic responses, delivery of oral vaccines in solution may not be optimal and targeting of antigens to the distal small intestine or large intestine may be more effective. This preferential induction of regulatory T cells in the proximal intestine could also be affected by the presence of adjuvants or the nature of the orally administered antigen. Vaccines could thus potentially over-ride the tolerogenic environment in the proximal intestine by inducing an inflammatory signature to allow the induction of effector T cell responses, although overall there may be an advantage in targeting the distal intestine.
One such large intestine-targeted oral delivery system was described where vaccine nanoparticles were delivered within pH-dependent microparticles. This oral construct induced protective antiviral immunity in the rectum and vagina comparable with levels seen with colorectal vaccination and protected against rectal and vaginal viral challenge 43 , providing a potentially productive route for mucosal vaccination for STDs. Although compartmentalization of mucosal immune responses is well established, confirming that the same subdivisions and connections apply from rodents to humans can be a challenge. However, it was shown that oral Dukoral vaccination increased the numbers of circulating IgA + memory B cells with a surface marker expression profile indicative of homing to the large intestine and respiratory tract 38 . Furthermore, translation of vaccine delivery strategies from small animal models to humans can be challenging owing to differences in parameters including gastrointestinal pH, gastrointestinal tract residence times and intestinal surface area. Some of these factors have been addressed in the case of oral drug delivery, but it is quite clear that in the absence of immunogenic antigens and effective adjuvants, addressing delivery challenges in isolation offers modest benefits and the vaccine components must be optimized for the targeted mucosal pathogen and its site of infection. The nature of the antigen is also a major determinant of responses, whether living or non-living, soluble or particulate. This can dictate the nature of antigen uptake pathways at mucosal sites and should be a principal design feature in the development of mucosal vaccines (Fig. 3). Particulate antigens — whether as whole bacterial cells, attenuated or inactivated viruses, virus-like particles or synthetic particulate formulations — are more immunogenic than purified proteins 33 and, in addition to their greater inherent immunogenicity, when delivered mucosally their particulate nature can impact on the site of uptake and APC targeting.
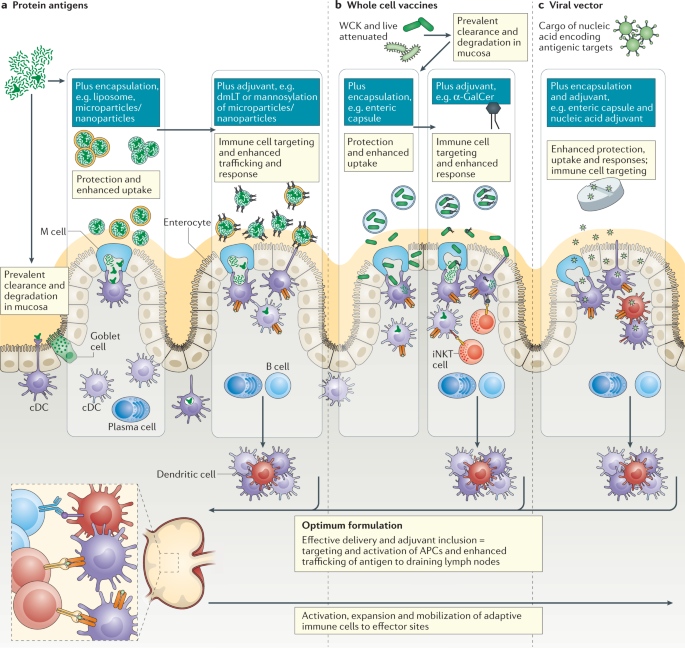
Nature of antigen uptake in the intestine is dependent on the type of vaccine components used, whether soluble or particulate, inert or live. Innovative encapsulation and targeting strategies have the potential to protect antigens while enhancing uptake and delivery to optimal intestinal regions. Inclusion of an effective mucosal vaccine adjuvant can confer multiple benefits from preventing tolerogenic responses to antigens, recruiting and activating antigen-presenting cells (APCs) and engaging other innate immune cells that contribute to protective immunity. Although there are currently few safe and effective adjuvants, cell-targeting adjuvants such as Escherichia coli double-mutant heat-labile toxin (dmLT), which binds to gangliosides on microfold (M) cells and dendritic cells, or α-galactosylceramide (α-GalCer), which can promote activation of invariant natural killer T (iNKT) cells locally and in draining lymph nodes via dendritic cell-mediated presentation, offer promise. Optimal formulations will address antigen design, adjuvanticity and antigen protection and targeting to address the unique challenges of intestinal delivery in the case of protein antigens (part a), whole cell vaccines (part b) and viral vector vaccines (part c). cDC, conventional dendritic cell; WCK, whole-cell killed.
Antigen-presenting cells in mucosal tissues
APC populations at mucosal sites are highly dynamic. In addition to tissue-resident dendritic cells and macrophages, during inflammatory responses or infection, further APCs are recruited that can engage with absorbed antigen and contribute to effector responses 44 . Indeed, there is evidence that during inflammation, monocytes in the gut and lungs can upregulate CCR7, migrate to lymph nodes and prime T cell responses 45 , and recruited monocytes or immature macrophages can produce inflammatory cytokines that contribute to T helper 1 (TH1) cell and TH17 cell responses 44 . Local inflammation triggered by mucosal vaccines and/or adjuvants could thus contribute to enhanced adaptive immunity by recruiting APCs. Although tissue-resident macrophages do not migrate to lymph nodes and are thus unlikely to directly prime T cells, antigen sampling CX3C-chemokine receptor 1 (CX3CR1 + ) mononuclear phagocytes can transfer antigen to dendritic cells 46 (Fig. 3) and colonic CX3CR1 + mononuclear phagocytes were shown to be required for induction of TH17 cell and antibody responses to intestinal fungi 47 .
Two key dendritic cell populations in gut-draining lymph nodes — CD103 + CD11b – dendritic cells and CD103 + CD11b + dendritic cells — have been associated with tolerogenic or pro-inflammatory immune responses, respectively 48 . Assigning specific roles to dendritic cell and macrophage populations in the intestine can be challenging as this is context-dependent. A recent study in a model of S. Typhimurium infection found that intestinal CX3CR1 + macrophages were superior to conventional type 1 dendritic cell (cDC1) and cDC2 populations in promoting the production of S. Typhimurium-specific IgA 49 . Furthermore, these broad categories of dendritic cell populations may not capture the true complexity of responses in the intestine, and subsets of these populations can contribute to the recruitment and activation of T cells and B cells at the site of infection. Differential gene expression profiles in cDC1 and cDC2 populations from different gut regions were reported 42 , indicating that not only the type of gut dendritic cell but also its precise tissue location may be key for the outcome of oral vaccination. The latter study also found that, compared with proximal gut-draining lymph nodes, distal gut-draining lymph nodes are more efficient in promoting the differentiation of TH17 cells. Given the importance of TH17 cells for defence against extracellular pathogens and for support of intestinal IgA secretion, this finding may be instructive for delivery of oral vaccines. It is of note that cDC1 and cDC2 frequencies remain relatively stable throughout life 50 (Table 1), an important consideration for cDC-targeted vaccine strategies. In the nasal mucosa, resident cDCs are vital for maintaining non-responsiveness to harmless inhaled antigens but viral infection promotes recruitment of monocyte-derived dendritic cells (moDCs) that can mediate T cell priming 51 . This study utilized a nasally administered chitosan hydrogel vaccine platform to trigger the inflammatory recruitment of moDCs coupled to the sustained release of antigen, which successfully promoted antigen-specific CD8 + T cell activation and expansion. This suggests that in addition to potentially targeting specific populations of mucosal dendritic cells, vaccines can also aim to promote recruitment of monocytes and moDCs to mediate protective mucosal immunity. Indeed, with subunit antigens, adjuvants may be critical to overcome tolerance induction and may also contribute to recruitment of ‘unconditioned’ APCs that prime antigen-specific T cells and B cells. Assessing the capacity of mucosal adjuvants to alter the activation states of tissue-associated or lymph node dendritic cells and to recruit monocytes and other APCs may be a useful indicator of efficacy, certainly more so than in vitro screening of cultured dendritic cells or macrophages that may poorly reflect responses of mucosal APCs following vaccination.
Induction of IgA and other mucosal antibodies
In terms of correlates of immunity following mucosal vaccination, induction of antigen-specific IgA is a crucial consideration. IgA is the dominant antibody at many mucosal sites and mediates protection against a range of enteropathogens. Recently, the importance of dimeric IgA in neutralization of SARS-CoV-2 has been highlighted in patients infected with the virus 52 and high-avidity IgA can protect against enteropathogens by processes including agglutination and the recently described process of ‘enchained growth’ 53 . For oral vaccination with adjuvanted subunit antigens, multiple doses (at least three) are needed to induce effective secretory IgA responses 21 . Induction of antigen-specific IgA was observed following two doses of ETVAX, with the addition of the E. coli double-mutant heat-labile toxin (dmLT) adjuvant enhancing the kinetics of induction 31 . This suggests that suitable adjuvants may exert a dose-sparing effect in mucosal IgA induction. Vaccines utilizing viral vectors and attenuated viruses may induce IgA responses more efficiently — for example, one dose of intranasal live attenuated influenza vaccine can elicit mucosal IgA 54 in recipients, and faecal/salivary IgA was observed in recipients of the Vaxart norovirus vaccine candidate, comprising an adjuvanted enterically stable adenovirus type 5 (Ad5) vector 55 . Although detailed assessment of antigen-specific mucosal immunity is more challenging in humans than in preclinical models, recent advances are facilitating novel means of determining vaccine-induced mucosal IgA responses in clinical trials (Box 2).
Although the importance of mucosal dendritic cell-mediated antigen sampling and trafficking to draining lymph nodes for induction of IgA responses has long been appreciated, Komban et al. recently uncovered a new layer of antigenic crosstalk between microfold (M) cells and B cells in the subepithelial dome region of Peyer’s patches. CC26 + CCR1 + GL7 – B cells were shown to be capable of sampling antigen directly from M cells and trafficking to germinal centres where their activation and population expansion occurs, challenging the idea of dependence on cDC-mediated antigen transfer for optimal antigen-specific IgA induction 56 . However, whereas transport of secretory antibody (IgA, IgM) via the polymeric immunoglobulin receptor is an essential and highly efficient process in the intestine, this is not the case at all mucosal sites. In the female reproductive tract, IgG rather than IgA can be critical for protective immunity to viral infection 57 . Likewise, IgG plays an important role in protective immunity in the lower respiratory tract whereas IgA is relatively more important in the nasal compartment 58 .
Box 2 Methodologies and challenges in studying human mucosal immune responses following vaccination
Although mucosal immune responses can be characterized preclinically in great detail in tissues and secretions, this is challenging in a clinical context. Therefore, establishing robust correlates of vaccine-induced adaptive immunity is a priority 156,157,158 . Assessing antibody responses in mucosal secretions has been a predominant approach. Indeed, vaccine-induced IgA responses in saliva 159 , nasal wash 160 and faecal samples 161 are frequently determined. Salivary IgA sampled from the submandible/sublingual region has also been shown to correlate well with intestinal IgA responses in an enterotoxigenic Escherichia coli (ETEC) challenge study 162 . The potential to determine mucosal cellular immune responses is restricted by access to tissues, and as sampling from mucosal sites such as the lungs and intestines is invasive and unpleasant for trial volunteers, blood collection is predominantly relied upon to identify vaccine-induced migrating effector cells in peripheral blood mononuclear cells. Circulating mucosal effector cell populations can be characterized using lineage and effector markers, alongside mucosal homing marker expression by flow cytometry. ELISPOT assays can be used to measure antibody-secreting cells 163 in isolated peripheral blood mononuclear cells; alternatively, supernatants from cultured peripheral blood mononuclear cells can be harvested for evaluation of antibodies in lymphocyte secretion (antibody in lymphocyte supernatant (ALS)) 164 by ELISA or multiplex assays. As the circulation of mucosa-derived lymphocytes in the blood is a dynamic and transient process, optimization of kinetics is critical. Assessing responses 5 days following oral booster vaccination has been suggested as optimal for detecting antibody-secreting cells/ALS responses 161 , which have been shown to correlate with mucosal immune responses in the case of challenge or vaccination trials with ETEC and cholera vaccines 165,166,167,168 . Logistically, ALS methodologies are advantageous as supernatants can be frozen for later analysis. Mottram et al. 169 recently identified B cell maturation antigen (BCMA) as a biomarker for the induction of vaccine-specific IgA and memory B cell responses to multiple antigens when measured via ELISA in ALS samples following oral vaccination; this simplified assay may prove especially useful when only low blood volumes are available, for example, in paediatric samples.
Tissue-resident memory T cells in mucosal tissues
Tissue-resident memory T (TRM) cells have been identified at multiple mucosal sites 59 and are thought to play decisive roles in rapid responses to infection 60 and cancers (Box 3), thus providing another important correlate for mucosal vaccine efficacy. The human duodenal CD4 + T cell compartment was recently shown to be enriched with a population of polyfunctional TH1 cells, which survive for at least 1 year 61 . This offers significant hope for inducing sustained protective cellular immunity if optimal oral vaccine strategies are designed. There are clearly significant tissue-specific differences in the nature of TRM cell populations so this must be considered in the design of new vaccine approaches. CD8 + TRM cells in the lungs are pivotal for protection against respiratory viral infections but these cells are generally relatively short-lived, and this can compromise responses to subsequent infection 62 . The latter study found that systemic vaccination (intravenous administration of Listeria monocytogenes expressing influenza virus-associated antigen) could enhance lung TRM cells in mice previously infected with influenza virus by increasing numbers of circulating effector memory T cells. This clearly has implications regarding the potential for systemic booster vaccinations in previously infected or mucosally primed populations to sustain resident memory CD8 + T cells in the lungs. A population of lung-resident helper T cells was recently characterized that was required to support tissue-resident memory B cells and CD8 + cells following influenza virus infection 63,64 . These cells, which are induced locally in the lung, may be key for promoting long-lived cellular and humoral immunity following vaccination in the respiratory tract, so the optimal strategy for their induction should be addressed. Recent evidence indicates that long-term maintenance of lung TRM cells requires airway vaccination and sustained antigen presence in the lungs, which was facilitated by an adenovirus vector vaccine 65 . It was recently shown in mouse models that TRM cells migrate to the mediastinal lymph nodes from the lungs during infection in a process termed ‘retrograde migration’. These cells retained a TRM cell phenotype and provided long-term protection 66 . This may be an important consideration following intranasal vaccination strategies. Further studies from the same group demonstrate that, upon restimulation, TRM cells can undergo retrograde migration and give rise to effector memory T cells and central memory T cells that have a predisposition for homing to their tissue of origin 67 .
Box 3 Vaccine approaches for mucosal cancers
Malignancies commonly emerge at the mucosae, providing a rationale for mucosal vaccine targeting. Incidences of mucosal cancers are increasing, with cancers of the head and neck and the reproductive, respiratory and digestive tracts estimated to cause 8.52 million deaths per annum by 2040 compared with a current estimate of 5.15 million deaths per annum 170 . Effective tumour immunosurveillance and elimination relies on tumour-specific CD8 + T cells. Therefore, mucosal vaccine strategies that effectively mobilize cell-mediated immunity with generation of sentinel tissue-resident memory T cells (TRM cells) are required 171,172 . Mucosal vaccination strategies have proved more effective than parenteral vaccination routes 171,173,174 . Nizard et al. demonstrated that an intranasal dendritic cell-targeted vaccine was more protective than parenteral vaccination in an orthotopic head and neck tumour model (HPV16 E6 + and E7 + expressing TC-1), and this effect was dependent on the presence of mucosal antigen-specific CD8 + TRM cells 171 . Optimal targeting of dendritic cell subsets is vital for such approaches: conventional type 1 dendritic cell (cDC1) populations including CD103 + non-lymphoid dendritic cells (analogous to CD141 + dendritic cells in humans) and CD8α + lymphoid dendritic cells are efficient at cross-priming cytotoxic T lymphocytes 175 and in imprinting mucosal homing receptors, displaying antitumour functionality 176,177 . Furthermore, the proficiency of CD103 + dendritic cells in trafficking intact antigens from tumours to tumour-draining lymph nodes and their importance in the context of checkpoint blockade responses has been highlighted 176,178,179 . Vaccines/adjuvants that effectively target these subsets and/or strategies to expand their number prior to vaccination should prove most successful. With the exception of virally induced cancers where viral antigens are vaccine targetable, antigen selection is problematic in prophylactic vaccination. Targeting tumour neoantigens is an attractive concept yet it is unlikely to ever be a ‘one size fits all approach’ as the degree and composition of mutational burden is highly patient-specific and tumour-specific 180,181 . Therapeutic mucosal cancer vaccines can circumvent these issues via personalized medicine-based vaccine design 182,183 or, possibly, through local antigen release for a more general approach 184 . Mucosal cancer vaccines not only have potential to prevent and treat mucosal tumours, but also to prevent infection with viruses linked to non-mucosal malignancies, such as Epstein–Barr virus and hepatitis viruses.
Targeting the genital tract
Although oral, sublingual and nasal routes are more convenient and there are currently no vaccines that specifically target the genital tract in clinical use, vaccination in the genital tract could have significant advantages in targeting STDs, even as a vaccine-boosting approach. In mouse models, vaginal immunization with herpes simplex virus 2 (HSV-2) glycoprotein D antigen and the adjuvant α-galactosylceramide (α-GalCer) induced protective immunity against HSV-2 challenge 68 . A combined vaccination approach using recombinant influenza virus–HIV vectors administered via intranasal and intravaginal routes (in mice) resulted in HIV-specific CD8 + TRM cells in the vaginal mucosa 69 . Vaginal immunization of mice with an attenuated HSV-2 strain resulted in the induction of a population of IFNγ + CD4 + TRM cells, which promoted CXCL9-mediated and CXCL10-mediated recruitment of memory B cells upon secondary challenge 70 . By contrast, primary vaccination did not result in the induction of a tissue-resident population of plasma cells in the female reproductive tract. Thus, vaginal booster vaccination or, possibly, booster vaccination in the large intestine may be an effective strategy following systemic priming to trigger genital tract responses, although these findings must first be confirmed in a human setting. Promising recent data showed that vaginal delivery (by intramucosal vaginal injection or spray) of recombinant glycosylated IL-7 to rhesus macaques acted as an effective mucosal adjuvant, enhancing the induction of antigen-specific IgA/IgG in the vaginal mucosa following subsequent vaginal delivery of diphtheria toxoid 71 . This could be a broadly applicable strategy that may overcome hypo-responsiveness to vaginal vaccine delivery.
Immune cell populations targeted by mucosal vaccines
Mucosal adjuvants should aim to activate and target local or recruited APCs (Fig. 3) or populations of immune cells enriched in the mucosa (Table 1) in order to mount effective mucosal responses. Innate lymphoid cells (ILCs), mucosal-associated invariant T cells, natural killer T (NKT) cells and γδ T cells are abundant in mucosal tissues and can play crucial roles in mediating and shaping mucosal immunity 72,73,74,75,76,77,78,79 . Adjuvants can also be exploited in parenteral–mucosal push–pull strategies; for example, dmLT has been shown to imprint mucosal homing markers on T cells when injected 80 . Similarly, retinoic acid has been identified as a suitable adjuvant in such strategies, imprinting gut-homing markers on T cells and leading to protective intestinal responses following subcutaneous vaccination 81 . There are currently two ongoing trials investigating parenteral–mucosal push–pull strategies for SARS-CoV-2 vaccination: NCT04732468 and IG/VPIN/CVD19/2001. The former trial involves investigating combinations of oral and subcutaneous immunization with a human adenoviral vector expressing modified SARS-CoV-2 spike and nucleocapsid proteins. By contrast, the latter trial involves combinations of intranasal and intramuscular immunization, with the vaccine composed of the receptor-binding domain of SARS-CoV-2 spike protein adjuvanted with hepatitis B virus nucleocapsid protein when given intranasally and with alum when given intramuscularly.
Mucosal adjuvant approaches
Enhancing the efficacy of subunit and inactivated antigens
Toxoid adjuvants are the best-characterized class of mucosal adjuvants and the development of safe yet potent derivatives of E. coli heat-labile toxin and cholera toxin (Box 4) has paved the way for their safe incorporation in vaccine formulations. Incorporation of dmLT has been shown to improve clinical responses to several whole-cell antigens, as seen with ETVAX and ACE527 (refs 31,82 ). Excellent overviews of the development and clinical application of dmLT are provided by Clements and Norton 80 and Qadri et al. 31 . Based on a similar approach, the adjuvant multiple mutated cholera toxin (mmCT) has been proposed as an alternative to dmLT 83 , and in preclinical studies mmCT has been shown to enhance TH1 cell and TH17 cell responses in addition to mucosal and serum antibodies to a whole-cell Helicobacter pylori antigen 84 . CTA1DD is a cholera toxin-derived adjuvant that was designed to combine the beneficial immunostimulatory effects of the CTA subunit enzyme with the B cell-targeting properties of a D-domain dimer from Staphylococcus aureus, to reduce off-target effects and toxicities 85,86 . It was recently shown that CTA1DD enhanced the maturation of follicular dendritic cells in lymph nodes following mucosal vaccination in neonatal mice and that oral priming with a construct incorporating the influenza virus M2e antigen (CTA1-3M2e-DD) induced protective immunity in neonates against influenza challenge 87 . Combination of lipid nanoparticles and CTA1-3M2e-DD generated a highly effective nasal vaccination system that conferred protective immunity against influenza virus infection in mice 88 . This combination adjuvant was particularly effective in promoting respiratory tract IgA, TH1 cell and TH17 cell responses, holding promise for universal influenza vaccination applications 88 . However, the efficacy and safety of CTA1DD remains to be determined clinically. The use of toxoid adjuvants intranasally has been somewhat marred by the clinical emergence of Bell’s palsy in some recipients of influenza vaccines adjuvanted with wild-type E. coli heat-labile toxin 89 or LTK63, a genetically detoxified E. coli heat-labile toxin derivative 90 . An alternative is sublingual vaccination, which has shown significant promise as a means of promoting protective immunity in animal models 91,92 although immune responses to sublingual dmLT were modest in a clinical trial 93 . There may be scope to enhance such responses by formulation with agents such as chitosan to enhance antigen and adjuvant residence times (Box 2).
These studies would suggest that nasal delivery of ganglioside-targeting toxoid adjuvants is inadvisable. However, results from a phase II clinical trial on a trivalent influenza vaccine, composed of haemagglutinin and adjuvanted with LThαK, a detoxified E. coli heat-labile toxin derivative, were recently reported 94,95 (NCT03784885). An acceptable safety profile was reported following two nasal vaccinations, which induced higher antigen-specific nasal IgA responses than the non-adjuvanted antigen 94 . LThαK is reported to have no ribosylating activity, correlating with enhanced retention in the nasal passages and the enhanced safety profile. Phase III trials will investigate efficacy in a challenge setting and will further elucidate the safety of LThαK for intranasal incorporation in a larger patient cohort. Importantly, nasal delivery of CTA1DD did not result in trafficking to the olfactory bulb, indicating its safety as a nasal vaccine adjuvant 88 . In summary, toxoid adjuvants are the most advanced mucosal adjuvants, having demonstrated impressive efficacy in clinical trials for oral whole-cell killed vaccines.
Aside from toxoid adjuvants, there are a small number of mucosal adjuvants with demonstrated safety and efficacy. The invariant NKT cell activator α-GalCer is a promising mucosal adjuvant and potentially an indicator of the potential for targeting innate-like lymphocytes to produce a new generation of mucosal adjuvants. We have demonstrated that oral delivery of a whole-cell killed H. pylori antigen adjuvanted with α-GalCer induced protective immunity from gastric bacterial challenge, characterized by induction of local IgA and TH1 cell immunity, comparable with a cholera toxin adjuvanted vaccine 96 . The induction of antigen-specific TH1 cell responses was dependent on CD1d, IL-1R1 and IL-17R signalling; therefore, α-GalCer provides a proof of principle for targeting the relatively abundant mucosal invariant NKT cell populations for effective adjuvanticity. We have further characterized α-GalCer as an effective adjuvant with oral whole-cell killed ETEC and cholera antigens including the CFA/I overexpressing JT-49 ETEC vaccine combined into enterically stable smPill mini-spheres. Potent induction of intestinal CFA/I-specific IgA was observed in addition to serum IgG responses 97 . Oral vaccination with Dukoral and α-GalCer induced stronger intestinal IgA and serum IgG responses than Dukoral alone and was comparable with cholera toxin adjuvanted Dukoral 98 . Finally, incorporation of α-GalCer in a novel multi-antigen cholera vaccine composed of whole-cell killed double-lipopolysaccharide (LPS) antigen expressing cholera vaccine with CTB promoted robust mucosal immunity with concomitant systemic antibody production, outperforming Dukoral and the whole-cell killed cholera alone 98 . Our preclinical data provide a rationale for the inclusion of α-GalCer in future whole-cell oral vaccines, which may lead to more durable protection, addressing shortcomings in immunity and response rates.
Chitosan is well established as a mucosal adjuvant/delivery system given its mucoadhesive properties and immunostimulatory effects 99 . We have demonstrated the effectiveness of chitosan as an intranasal adjuvant in mouse models. Intranasal vaccination with chitosan and pneumococcal surface protein A (PspA) led to the induction of lung PspA-specific IFNγ and IgG1, IgG2c and IgA responses that were dependent on STING signalling 100 . STING-activating cyclic dinucleotides have been trialled as mucosal adjuvants. An intranasal synthetic cyclic dinucleotide (cyclic diguanylate) adjuvanted subunit vaccine induced protective immunity against M. tuberculosis in mice, correlating with potent induction of TH17 cells 101 . Other cyclic dinucleotides — including cyclic di-AMP and cyclic di-GMP — have also shown promise as mucosal adjuvants 102,103 . Mansouri et al. recently highlighted roles for two lung cDC2 populations in intranasal cyclic di-GMP adjuvanticity. Antibody responses were dependent on activation of moDCs by TNFR2 – cDC2 populations, with subsequent T follicular helper cell and germinal centre B cell activation, whereas induction of TH1 cell and/or TH17 cell responses was dependent on their TNFR + cDC2 counterparts 104 . These studies provide a strong rationale for further development of mucosal adjuvants targeting the STING pathway. In this context, chitosan has specific advantages in its record of clinical use and mucoadhesive properties in addition to STING-dependent adjuvanticity.
Whereas emulsion-based adjuvants have been highly successful in injectable vaccines, such approaches have not reached clinical application mucosally. Bluewillow Biologics currently have a phase I trial underway (NCT04148118) utilizing an intranasally administered nanoemulsion (oil in water emulsion) adjuvanted recombinant protein vaccine against anthrax (BW-1010). Preclinically, this vaccine has previously been shown to be protective in guinea pig models of infection, correlating with induction of systemic and local antibody induction 105 . The nanoemulsion adjuvant has also been shown to promote TH1 cell immunity and TH17 cell immunity in anthrax and M. tuberculosis vaccine formulations, respectively 105,106 . Kimoto et al. recently reported a promising mucosal adjuvant with possible applications in oral and intranasal vaccination routes. Two oral doses of HAv (haemagglutinin-based vaccine) adjuvanted with SF10 (a synthetic surfactant adjuvant) led to induction of antigen-specific mucosal IgA and protection from influenza in a preclinical model, outperforming cholera toxin 107 . These recent studies highlight the promise of adjuvanted mucosal vaccines, with many taking inspiration from bacteria-derived virulence factors and showing promise for inclusion not only in subunit but also whole-cell formulations.
Box 4 Enterotoxin-derived mucosal adjuvants: dmLT and mmCT
Cholera toxin and Escherichia coli heat-labile toxin are the gold-standard mucosal adjuvants. However, their toxicity necessitated strategies to enhance safety whilst retaining adjuvanticity, culminating in generation of E. coli double-mutant heat-labile toxin (dmLT) 185 and multiple-mutated cholera toxin (mmCT) 83 . The introduced mutations target the ADP ribosyltransferase activity of the toxin A subunit 83,185 and both molecules are powerful mucosal adjuvants that enhance mucosal IgA and serum IgG responses in addition to CD4 + T cell responses, particularly T helper 17 (TH17) cells 80,84 . Interaction of cholera toxin with GM1 on gut dendritic cells is required for its oral adjuvanticity 146 , although the precise mechanisms of action are not fully resolved. NF-κB activation was required for the adjuvanticity of mmCT, with cyclic AMP–protein kinase A (cAMP–PKA) signalling proposed to be required for NF-κB activation in mmCT-stimulated dendritic cells in vitro 186 , although PKA may be dispensable for dendritic cell activation by dmLT 187 . Both dmLT and mmCT required cAMP–PKA-dependent inflammasome activation to promote human TH17-type responses 188 . There has been extensive evaluation of dmLT in clinical trials as an oral vaccine adjuvant, with results indicating an acceptable safety profile and strong adjuvanticity 31,32,80,82 . Furthermore, a National Institute of Allergy and Infectious Diseases (NIAID)-sponsored trial investigating the safety and adjuvanticity of three doses of dmLT by oral, sublingual and intradermal routes has begun recruitment in an enterotoxigenic E. coli (ETEC) endemic area of Bangladesh (NCT03548064). The inclusion of toxoid-derived adjuvants in mucosal vaccines may improve responses in low-responding demographics, such as older people and young children 31 . Toxoid-derived adjuvants may also potentially help in addressing the lower responses to oral vaccines that are often seen in endemic regions (known as the ‘tropical barrier’) compared with the higher income countries where early-stage clinical trials are frequently conducted. Although existing antibodies to dmLT do not appear to impair its adjuvanticity upon booster vaccination 80 , different outcomes have been observed in trials between Swedish and Bangladeshi recipients 31,32 that require further evaluation on the potential impact of previous exposure.
Mucosal nucleic acid and viral vectored vaccines
Until very recently, there were no licensed nucleic acid vaccines for clinical use. However, mRNA vaccines against SARS-CoV-2 have now been successfully trialled and rolled out for parenteral vaccination, displaying impressive efficacy and paving the way for others to follow 108 . Mucosal vaccination utilizing nucleic acids poses a greater challenge, as successful candidates must penetrate the mucus layer, translocate into target cells and evade extracellular and intracellular degradation. Vaccination via the oral route poses an added challenge with the low gastric pH and difficulty in ensuring release of the nucleic acid payload at the appropriate location. Innovative protective delivery strategies for nucleic acids have been developed using nanocarriers and biomaterials 109,110,111 , and in particular the complexing of nucleic acids with polycationic materials including chitosan and polyethylenimine (PEI) and encapsulation of the nucleic acid cargo utilizing liposomes and polymersomes are showing potential. Lipidoid nanoparticles have been shown to effectively deliver small interfering RNA molecules to intestinal epithelial cells in the lower small intestine and colon following oral administration 112 . Additionally, intranasal delivery of chitosan nanoparticles encapsulating mRNA with a viral protein coating elicited protection from avian influenza in chickens 113 . The coming years are likely to see great activity in this space, particularly around mobilizing solid lipid nanoparticles for mucosal RNA vaccine development.
Viral vectors are among the most promising strategies for mucosal vaccination, owing to their capacity for intracellular delivery, versatility and intrinsic immunogenicity. Viral vector strategies are applicable to oral vaccination when protection from conditions of the gastrointestinal tract and effective release are addressed. This is exemplified by the technology from Vaxart, whose oral influenza vaccine candidate VXA-A1.1 utilizes an enterically stable tableted delivery system, carrying a cargo of haemagglutinin encoding adenoviral vectors and a double-stranded RNA adjuvant. Data from phase I (NCT01688297) and phase II (NCT02918006) clinical trials demonstrated that VXA-A1.1 is well tolerated 114 and, crucially for future adenoviral vector strategies, is not hindered by pre-existing adenoviral immunity when given orally 114 . Oral vaccination with VXA-A1.1 induced superior protection from influenza A virus challenge compared with the conventional intramuscularly delivered FluZone vaccine 115 . Whether this platform can be used to develop an effective oral quadrivalent influenza vaccine remains to be demonstrated. Additionally, orally administered RSV vaccines are in preclinical development (VXA-RSV-f) 116 , and an oral Norovirus vaccine (VXA-G1.1-NN) showed favourable safety and immunogenicity in a phase 1 trial (NCT02868073) 55 . More recently, an orally administered SARS-CoV-2 vaccine (VXA-CoV2-1) was described that uses the same formulation and is currently in phase I trials (NCT04563702). The efficacy of the double-stranded RNA adjuvant included in this platform is also very promising as an alternative to the canonical toxoid-based adjuvants in various stages of development and broadens the range of PRR targets that can be exploited for oral vaccination. This double-stranded RNA adjuvant will likely effectively target dendritic cell populations for activation owing to their high TLR3 expression. This capacity to successfully adjuvant viral vectors may be critical as they will likely be less effective when given mucosally compared with parenteral routes. Further to this point, it has been recently shown that responses to viral vector (MVA) antigens can be enhanced by the saponin-containing adjuvant matrix M following subcutaneous vaccination 117 .
Viral vector approaches also hold potential for vaccination in the respiratory tract. Intranasal vaccination of mice with an adenoviral vector encoding influenza virus nucleoprotein induced a population of CD8 + TRM cells in the lungs that was sustained for longer than 1 year 65 . This was dependent on respiratory vaccination and sustained antigen expression, and contrasted with the situation following parenteral influenza virus infection, where the local CD8 + TRM cell population was rapidly lost. The authors suggested that induction of robust local cellular immunity may address issues surrounding the reliance on systemic antibody responses to haemagglutinin associated with parenteral influenza vaccination 65 . Nasal delivery of chimpanzee adenoviral (ChAd) vectors may also have potential in SARS-CoV-2 vaccines. Nasal delivery of ChAd-SARS-CoV-2 expressing homotrimeric spike antigen induced promising results in a murine infection model (K18-hACE2 mice). A single dose provided protection from upper and lower respiratory tract infection, correlating with induction of neutralizing antibody titres in the serum and bronchoalveolar lavage alongside the induction of IFNγ + and granzyme B + CD8 + T cells 118 . Whether efficacy would be sufficient clinically with viral vectors alone is unclear but, as with oral delivery, there may be scope to enhance responses with appropriately targeted mucosal adjuvants. With viral (or bacterial) vectored vaccines, the capacity of vaccine-induced secretory antibody responses to compromise responses to booster vaccination must be considered 119 . However, data from preclinical models have shown that pre-existing intestinal immunity did not compromise efficacy of an oral experimental viral vectored rabies vaccine 120 . Furthermore, compelling recent clinical data found no detrimental effect of pre-existing influenza-specific nasal IgA responses on the efficacy of nasal live attenuated influenza virus vaccination in children 121 , and recently Janssen reported no clear impact of pre-existing immunity to their Ad26 vectored vaccine platform on efficacy following priming or boosting vaccinations 122 . This must be ascertained for each specific vaccine but, moving forwards, the availability of numerous mucosal viral vectors and adjuvant strategies would allow a heterologous prime–boost approach to overcome pre-existing immunity if required.
Concluding remarks
We are currently in the midst of a revolution in vaccine research and development. Cutting-edge research and advances into nucleic acid and viral vector vaccine technologies allowed SARS-CoV-2 vaccines to be developed and produced in an unprecedented short period of time. These advances are yet to impact on clinically used mucosal vaccines, but this will likely change in the near future. Mucosal vaccines offer the significant benefit of triggering immune responses at the principal sites of infection, offering scope for sterilizing immunity achieved by local secretory antibody responses and resident populations of CD4 + and CD8 + T cells. Thus, the outstanding obstacles to mucosal vaccine development are worth the effort as they are far outweighed by the potential immunological and logistical benefits in terms of ease of delivery. One of the major challenges that requires innovative solutions is the ‘tropical barrier’, where responses to oral vaccines in low- and middle-income countries can be lower than those seen throughout clinical trials in high-income countries. Interventions to address this problem are urgently required 123 and may include implementation of probiotic supplements prior to or during vaccination 124 . The potential for adjuvants to overcome suboptimal responses must be addressed as this and increased antigen doses may have a greater impact than other proposed strategies. Indeed, the most advanced mucosal adjuvant, dmLT, has demonstrated efficacy in both high-income and low-income countries 31,32 . Identifying whether other candidate adjuvants can also increase efficacy of existing oral vaccines as well as facilitating the development of novel vaccines is a priority. Targeting mucosally abundant cellular populations such as ILCs, mucosal-associated invariant T cells and NKT cells has significant promise but clinical validation of these approaches is required. A recent study in mice found that intestinal ILCs can migrate via the lymph to the mesenteric lymph nodes, and in response to infection with S. Typhimurium these migrating ILCs exhibited greater levels of activation and cytokine production. Mobilizing this population using ILC-targeting adjuvants may have significant potential to bolster mucosal immune responses 75 . In addition to stand-alone mucosal vaccine approaches, parenteral mucosal prime–boost strategies offer promise. These may be enhanced with injectable vaccines that imprint a degree of mucosal homing, for example, with dmLT 125 or retinoic acid 81 , and their relative ability to enhance tissue-resident T cell responses may be key to success. In some cases, antigen alone may be sufficient for mucosal boosting 126 although this will depend on the nature and immunogenicity of the antigen and it is likely, in most cases, that an effective adjuvant will be required. In summary, although the leaps forward in injectable vaccine strategies have not yet been seen with mucosal vaccines, this is likely to change in the near future. Advances in our understanding of mucosal protective immunity, developments in measuring human mucosal immunity 127 and antigen and adjuvant discovery offer hope that novel mucosal vaccines for infectious diseases and cancer are on the horizon.
mRNA vaccines — a new era in vaccinology
Recent improvements in mRNA vaccines act to increase protein translation, modulate innate and adaptive immunogenicity and improve delivery.
mRNA vaccines have elicited potent immunity against infectious disease targets in animal models of influenza virus, Zika virus, rabies virus and others, especially in recent years, using lipid-encapsulated or naked forms of sequence-optimized mRNA.
Diverse approaches to mRNA cancer vaccines, including dendritic cell vaccines and various types of directly injectable mRNA, have been employed in numerous cancer clinical trials, with some promising results showing antigen-specific T cell responses and prolonged disease-free survival in some cases.
Therapeutic considerations and challenges include scaling up good manufacturing practice (GMP) production, establishing regulations, further documenting safety and increasing efficacy.
Important future directions of research will be to compare and elucidate the immune pathways activated by various mRNA vaccine platforms, to improve current approaches based on these mechanisms and to initiate new clinical trials against additional disease targets.
Abstract
mRNA vaccines represent a promising alternative to conventional vaccine approaches because of their high potency, capacity for rapid development and potential for low-cost manufacture and safe administration. However, their application has until recently been restricted by the instability and inefficient in vivo delivery of mRNA. Recent technological advances have now largely overcome these issues, and multiple mRNA vaccine platforms against infectious diseases and several types of cancer have demonstrated encouraging results in both animal models and humans. This Review provides a detailed overview of mRNA vaccines and considers future directions and challenges in advancing this promising vaccine platform to widespread therapeutic use.
Vaccines prevent many millions of illnesses and save numerous lives every year 1 . As a result of widespread vaccine use, the smallpox virus has been completely eradicated and the incidence of polio, measles and other childhood diseases has been drastically reduced around the world 2 . Conventional vaccine approaches, such as live attenuated and inactivated pathogens and subunit vaccines, provide durable protection against a variety of dangerous diseases 3 . Despite this success, there remain major hurdles to vaccine development against a variety of infectious pathogens, especially those better able to evade the adaptive immune response 4 . Moreover, for most emerging virus vaccines, the main obstacle is not the effectiveness of conventional approaches but the need for more rapid development and large-scale deployment. Finally, conventional vaccine approaches may not be applicable to non-infectious diseases, such as cancer. The development of more potent and versatile vaccine platforms is therefore urgently needed.
Nucleic acid therapeutics have emerged as promising alternatives to conventional vaccine approaches. The first report of the successful use of in vitro transcribed (IVT) mRNA in animals was published in 1990, when reporter gene mRNAs were injected into mice and protein production was detected 5 . A subsequent study in 1992 demonstrated that administration of vasopressin-encoding mRNA in the hypothalamus could elicit a physiological response in rats 6 . However, these early promising results did not lead to substantial investment in developing mRNA therapeutics, largely owing to concerns associated with mRNA instability, high innate immunogenicity and inefficient in vivo delivery. Instead, the field pursued DNA-based and protein-based therapeutic approaches 7,8 .
Over the past decade, major technological innovation and research investment have enabled mRNA to become a promising therapeutic tool in the fields of vaccine development and protein replacement therapy. The use of mRNA has several beneficial features over subunit, killed and live attenuated virus, as well as DNA-based vaccines. First, safety: as mRNA is a non-infectious, non-integrating platform, there is no potential risk of infection or insertional mutagenesis. Additionally, mRNA is degraded by normal cellular processes, and its in vivo half-life can be regulated through the use of various modifications and delivery methods 9,10,11,12 . The inherent immunogenicity of the mRNA can be down-modulated to further increase the safety profile 9,12,13 . Second, efficacy: various modifications make mRNA more stable and highly translatable 9,12,13 . Efficient in vivo delivery can be achieved by formulating mRNA into carrier molecules, allowing rapid uptake and expression in the cytoplasm (reviewed in Refs 10,11). mRNA is the minimal genetic vector; therefore, anti-vector immunity is avoided, and mRNA vaccines can be administered repeatedly. Third, production: mRNA vaccines have the potential for rapid, inexpensive and scalable manufacturing, mainly owing to the high yields of in vitro transcription reactions.
The mRNA vaccine field is developing extremely rapidly; a large body of preclinical data has accumulated over the past several years, and multiple human clinical trials have been initiated. In this Review, we discuss current mRNA vaccine approaches, summarize the latest findings, highlight challenges and recent successes, and offer perspectives on the future of mRNA vaccines. The data suggest that mRNA vaccines have the potential to solve many of the challenges in vaccine development for both infectious diseases and cancer.
Basic mRNA vaccine pharmacology
mRNA is the intermediate step between the translation of protein-encoding DNA and the production of proteins by ribosomes in the cytoplasm. Two major types of RNA are currently studied as vaccines: non-replicating mRNA and virally derived, self-amplifying RNA. Conventional mRNA-based vaccines encode the antigen of interest and contain 5′ and 3′ untranslated regions (UTRs), whereas self-amplifying RNAs encode not only the antigen but also the viral replication machinery that enables intracellular RNA amplification and abundant protein expression.
The construction of optimally translated IVT mRNA suitable for therapeutic use has been reviewed previously 14,15 . Briefly, IVT mRNA is produced from a linear DNA template using a T7, a T3 or an Sp6 phage RNA polymerase 16 . The resulting product should optimally contain an open reading frame that encodes the protein of interest, flanking UTRs, a 5′ cap and a poly(A) tail. The mRNA is thus engineered to resemble fully processed mature mRNA molecules as they occur naturally in the cytoplasm of eukaryotic cells.
Complexing of mRNA for in vivo delivery has also been recently detailed 10,11 . Naked mRNA is quickly degraded by extracellular RNases 17 and is not internalized efficiently. Thus, a great variety of in vitro and in vivo transfection reagents have been developed that facilitate cellular uptake of mRNA and protect it from degradation. Once the mRNA transits to the cytosol, the cellular translation machinery produces protein that undergoes post-translational modifications, resulting in a properly folded, fully functional protein. This feature of mRNA pharmacology is particularly advantageous for vaccines and protein replacement therapies that require cytosolic or transmembrane proteins to be delivered to the correct cellular compartments for proper presentation or function. IVT mRNA is finally degraded by normal physiological processes, thus reducing the risk of metabolite toxicity.
Recent advances in mRNA vaccine technology
Various mRNA vaccine platforms have been developed in recent years and validated in studies of immunogenicity and efficacy 18,19,20 . Engineering of the RNA sequence has rendered synthetic mRNA more translatable than ever before. Highly efficient and non-toxic RNA carriers have been developed that in some cases 21,22 allow prolonged antigen expression in vivo (Table 1). Some vaccine formulations contain novel adjuvants, while others elicit potent responses in the absence of known adjuvants. The following section summarizes the key advances in these areas of mRNA engineering and their impact on vaccine efficacy.
Optimization of mRNA translation and stability
This topic has been extensively discussed in previous reviews 14,15 ; thus, we briefly summarize the key findings (Box 1). The 5′ and 3′ UTR elements flanking the coding sequence profoundly influence the stability and translation of mRNA, both of which are critical concerns for vaccines. These regulatory sequences can be derived from viral or eukaryotic genes and greatly increase the half-life and expression of therapeutic mRNAs 23,24 . A 5′ cap structure is required for efficient protein production from mRNA 25 . Various versions of 5′ caps can be added during or after the transcription reaction using a vaccinia virus capping enzyme 26 or by incorporating synthetic cap or anti-reverse cap analogues 27,28 . The poly(A) tail also plays an important regulatory role in mRNA translation and stability 25 ; thus, an optimal length of poly(A) 24 must be added to mRNA either directly from the encoding DNA template or by using poly(A) polymerase. The codon usage additionally has an impact on protein translation. Replacing rare codons with frequently used synonymous codons that have abundant cognate tRNA in the cytosol is a common practice to increase protein production from mRNA 29 , although the accuracy of this model has been questioned 30 . Enrichment of G:C content constitutes another form of sequence optimization that has been shown to increase steady-state mRNA levels in vitro 31 and protein expression in vivo 12 .
Although protein expression may be positively modulated by altering the codon composition or by introducing modified nucleosides (discussed below), it is also possible that these forms of sequence engineering could affect mRNA secondary structure 32 , the kinetics and accuracy of translation and simultaneous protein folding 33,34 , and the expression of cryptic T cell epitopes present in alternative reading frames 30 . All these factors could potentially influence the magnitude or specificity of the immune response.
Box 1: Strategies for optimizing mRNA pharmacology
A number of technologies are currently used to improve the pharmacological aspects of mRNA. The various mRNA modifications used and their impact are summarized below.
• Synthetic cap analogues and capping enzymes 26,27 stabilize mRNA and increase protein translation via binding to eukaryotic translation initiation factor 4E (EIF4E)
• Regulatory elements in the 5′-untranslated region (UTR) and the 3′-UTR 23 stabilize mRNA and increase protein translation
• Poly(A) tail 25 stabilizes mRNA and increases protein translation
• Modified nucleosides 9,48 decrease innate immune activation and increase translation
• Separation and/or purification techniques: RNase III treatment (N.P. and D.W., unpublished observations) and fast protein liquid chromatography (FPLC) purification 13 decrease immune activation and increase translation
• Sequence and/or codon optimization 29 increase translation
• Modulation of target cells: co-delivery of translation initiation factors and other methods alters translation and immunogenicity
Modulation of immunogenicity
Exogenous mRNA is inherently immunostimulatory, as it is recognized by a variety of cell surface, endosomal and cytosolic innate immune receptors (Fig. 1) (reviewed in Ref. 35). Depending on the therapeutic application, this feature of mRNA could be beneficial or detrimental. It is potentially advantageous for vaccination because in some cases it may provide adjuvant activity to drive dendritic cell (DC) maturation and thus elicit robust T and B cell immune responses. However, innate immune sensing of mRNA has also been associated with the inhibition of antigen expression and may negatively affect the immune response 9,13 . Although the paradoxical effects of innate immune sensing on different formats of mRNA vaccines are incompletely understood, some progress has been made in recent years in elucidating these phenomena.
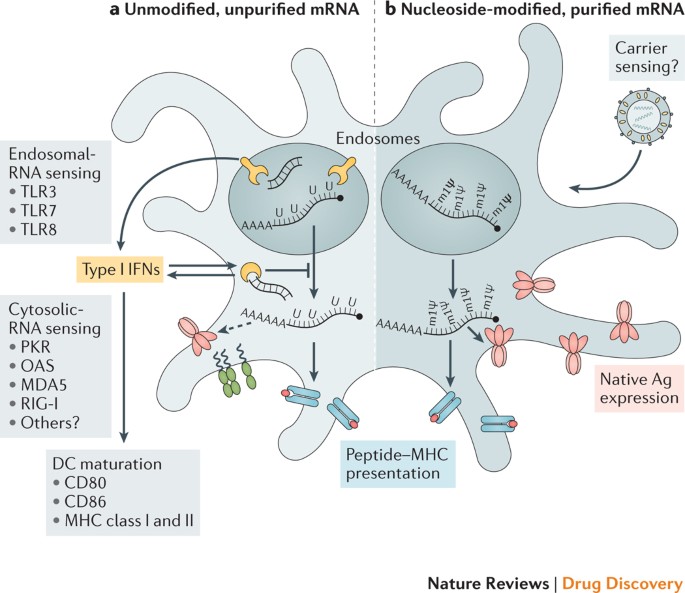
Innate immune sensing of two types of mRNA vaccine by a dendritic cell (DC), with RNA sensors shown in yellow, antigen in red, DC maturation factors in green, and peptide−major histocompatibility complex (MHC) complexes in light blue and red; an example lipid nanoparticle carrier is shown at the top right. A non-exhaustive list of the major known RNA sensors that contribute to the recognition of double-stranded and unmodified single-stranded RNAs is shown. Unmodified, unpurified (part a) and nucleoside-modified, fast protein liquid chromatography (FPLC)-purified (part b) mRNAs were selected for illustration of two formats of mRNA vaccines where known forms of mRNA sensing are present and absent, respectively. The dashed arrow represents reduced antigen expression. Ag, antigen; PKR, interferon-induced, double-stranded RNA-activated protein kinase; MDA5, interferon-induced helicase C domain-containing protein 1 (also known as IFIH1); IFN, interferon; m1Ψ, 1-methylpseudouridine; OAS, 2′-5′-oligoadenylate synthetase; TLR, Toll-like receptor.
Studies over the past decade have shown that the immunostimulatory profile of mRNA can be shaped by the purification of IVT mRNA and the introduction of modified nucleosides as well as by complexing the mRNA with various carrier molecules 9,13,36,37 . Enzymatically synthesized mRNA preparations contain double-stranded RNA (dsRNA) contaminants as aberrant products of the IVT reaction 13 . As a mimic of viral genomes and replication intermediates, dsRNA is a potent pathogen-associated molecular pattern (PAMP) that is sensed by pattern recognition receptors in multiple cellular compartments (Fig. 1). Recognition of IVT mRNA contaminated with dsRNA results in robust type I interferon production 13 , which upregulates the expression and activation of protein kinase R (PKR; also known as EIF2AK2) and 2′-5′-oligoadenylate synthetase (OAS), leading to the inhibition of translation 38 and the degradation of cellular mRNA and ribosomal RNA 39 , respectively. Karikó and colleagues 13 have demonstrated that contaminating dsRNA can be efficiently removed from IVT mRNA by chromatographic methods such as reverse-phase fast protein liquid chromatography (FPLC) or high-performance liquid chromatography (HPLC). Strikingly, purification by FPLC has been shown to increase protein production from IVT mRNA by up to 1,000-fold in primary human DCs 13 . Thus, appropriate purification of IVT mRNA seems to be critical for maximizing protein (immunogen) production in DCs and for avoiding unwanted innate immune activation.
Besides dsRNA contaminants, single-stranded mRNA molecules are themselves a PAMP when delivered to cells exogenously. Single-stranded oligoribonucleotides and their degradative products are detected by the endosomal sensors Toll-like receptor 7 (TLR7) and TLR8 (Refs 40,41), resulting in type I interferon production 42 . Crucially, it was discovered that the incorporation of naturally occurring chemically modified nucleosides, including but not limited to pseudouridine 9,43,44 and 1-methylpseudouridine 45 , prevents activation of TLR7, TLR8 and other innate immune sensors 46,47 , thus reducing type I interferon signalling 48 . Nucleoside modification also partially suppresses the recognition of dsRNA species 46,47,48 . As a result, Karikó and others have shown that nucleoside-modified mRNA is translated more efficiently than unmodified mRNA in vitro 9 , particularly in primary DCs, and in vivo in mice 45 . Notably, the highest level of protein production in DCs was observed when mRNA was both FPLC-purified and nucleoside-modified 13 . These advances in understanding the sources of innate immune sensing and how to avoid their adverse effects have substantially contributed to the current interest in mRNA-based vaccines and protein replacement therapies.
In contrast to the findings described above, a study by Thess and colleagues found that sequence-optimized, HPLC-purified, unmodified mRNA produced higher levels of protein in HeLa cells and in mice than its nucleoside-modified counterpart 12 . Additionally, Kauffman and co-workers demonstrated that unmodified, non-HPLC-purified mRNA yielded more robust protein production in HeLa cells than nucleoside-modified mRNA, and resulted in similar levels of protein production in mice 49 . Although not fully clear, the discrepancies between the findings of Karikó 9,13 and these authors 12,49 may have arisen from variations in RNA sequence optimization, the stringency of mRNA purification to remove dsRNA contaminants and the level of innate immune sensing in the targeted cell types.
The immunostimulatory properties of mRNA can conversely be increased by the inclusion of an adjuvant to increase the potency of some mRNA vaccine formats. These include traditional adjuvants as well as novel approaches that take advantage of the intrinsic immunogenicity of mRNA or its ability to encode immune-modulatory proteins. Self-replicating RNA vaccines have displayed increased immunogenicity and effectiveness after formulating the RNA in a cationic nanoemulsion based on the licensed MF59 (Novartis) adjuvant 50 . Another effective adjuvant strategy is TriMix, a combination of mRNAs encoding three immune activator proteins: CD70, CD40 ligand (CD40L) and constitutively active TLR4. TriMix mRNA augmented the immunogenicity of naked, unmodified, unpurified mRNA in multiple cancer vaccine studies and was particularly associated with increased DC maturation and cytotoxic T lymphocyte (CTL) responses (reviewed in Ref. 51). The type of mRNA carrier and the size of the mRNA–carrier complex have also been shown to modulate the cytokine profile induced by mRNA delivery. For example, the RNActive (CureVac AG) vaccine platform 52,53 depends on its carrier to provide adjuvant activity. In this case, the antigen is expressed from a naked, unmodified, sequence-optimized mRNA, while the adjuvant activity is provided by co-delivered RNA complexed with protamine (a polycationic peptide), which acts via TLR7 signalling 52,54 . This vaccine format has elicited favourable immune responses in multiple preclinical animal studies for vaccination against cancer and infectious diseases 18,36,55,56 . A recent study provided mechanistic information on the adjuvanticity of RNActive vaccines in mice in vivo and human cells in vitro 54 . Potent activation of TLR7 (mouse and human) and TLR8 (human) and production of type I interferon, pro-inflammatory cytokines and chemokines after intradermal immunization was shown 54 . A similar adjuvant activity was also demonstrated in the context of non-mRNA-based vaccines using RNAdjuvant (CureVac AG), an unmodified, single-stranded RNA stabilized by a cationic carrier peptide 57 .
Progress in mRNA vaccine delivery
Efficient in vivo mRNA delivery is critical to achieving therapeutic relevance. Exogenous mRNA must penetrate the barrier of the lipid membrane in order to reach the cytoplasm to be translated to functional protein. mRNA uptake mechanisms seem to be cell type dependent, and the physicochemical properties of the mRNA complexes can profoundly influence cellular delivery and organ distribution. There are two basic approaches for the delivery of mRNA vaccines that have been described to date. First, loading of mRNA into DCs ex vivo, followed by re-infusion of the transfected cells 58 ; and second, direct parenteral injection of mRNA with or without a carrier. Ex vivo DC loading allows precise control of the cellular target, transfection efficiency and other cellular conditions, but as a form of cell therapy, it is an expensive and labour-intensive approach to vaccination. Direct injection of mRNA is comparatively rapid and cost-effective, but it does not yet allow precise and efficient cell-type-specific delivery, although there has been recent progress in this regard 59 . Both of these approaches have been explored in a variety of forms (Fig. 2; Table 1).
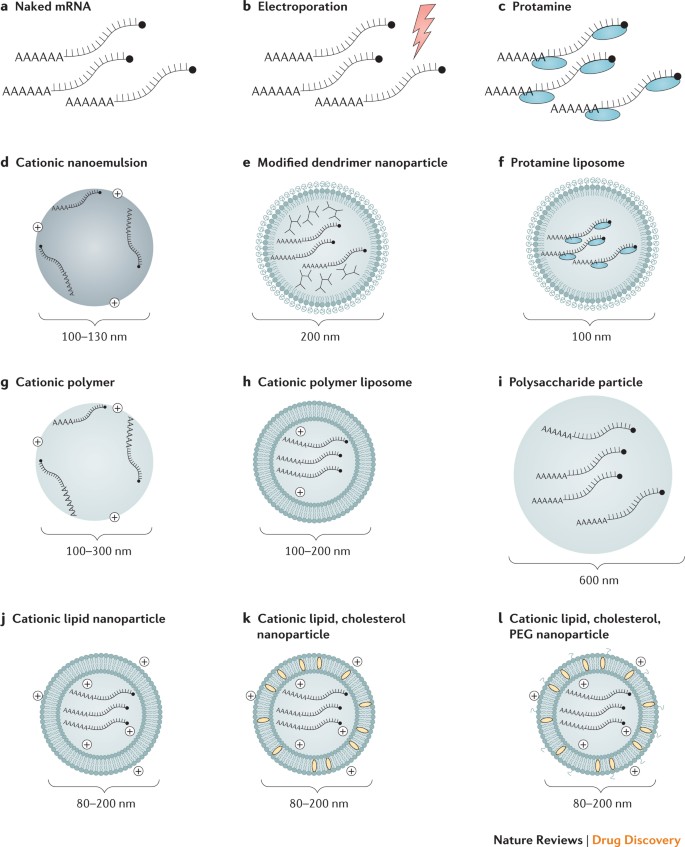
Commonly used delivery methods and carrier molecules for mRNA vaccines along with typical diameters for particulate complexes are shown: naked mRNA (part a); naked mRNA with in vivo electroporation (part b); protamine (cationic peptide)-complexed mRNA (part c); mRNA associated with a positively charged oil-in-water cationic nanoemulsion (part d); mRNA associated with a chemically modified dendrimer and complexed with polyethylene glycol (PEG)-lipid (part e); protamine-complexed mRNA in a PEG-lipid nanoparticle (part f); mRNA associated with a cationic polymer such as polyethylenimine (PEI) (part g); mRNA associated with a cationic polymer such as PEI and a lipid component (part h); mRNA associated with a polysaccharide (for example, chitosan) particle or gel (part i); mRNA in a cationic lipid nanoparticle (for example, 1,2-dioleoyloxy-3-trimethylammoniumpropane (DOTAP) or dioleoylphosphatidylethanolamine (DOPE) lipids) (part j); mRNA complexed with cationic lipids and cholesterol (part k); and mRNA complexed with cationic lipids, cholesterol and PEG-lipid (part l).
Ex vivo loading of DCs. DCs are the most potent antigen-presenting cells of the immune system. They initiate the adaptive immune response by internalizing and proteolytically processing antigens and presenting them to CD8 + and CD4 + T cells on major histocompatibility complexes (MHCs), namely, MHC class I and MHC class II, respectively. Additionally, DCs may present intact antigen to B cells to provoke an antibody response 60 . DCs are also highly amenable to mRNA transfection. For these reasons, DCs represent an attractive target for transfection by mRNA vaccines, both in vivo and ex vivo.
Although DCs have been shown to internalize naked mRNA through a variety of endocytic pathways 61,62,63 , ex vivo transfection efficiency is commonly increased using electroporation; in this case, mRNA molecules pass through membrane pores formed by a high-voltage pulse and directly enter the cytoplasm (reviewed in Ref. 64). This mRNA delivery approach has been favoured for its ability to generate high transfection efficiency without the need for a carrier molecule. DCs that are loaded with mRNA ex vivo are then re-infused into the autologous vaccine recipient to initiate the immune response. Most ex vivo-loaded DC vaccines elicit a predominantly cell-mediated immune response; thus, they have been used primarily to treat cancer (reviewed in Ref. 58).
Injection of naked mRNA in vivo. Naked mRNA has been used successfully for in vivo immunizations, particularly in formats that preferentially target antigen-presenting cells, as in intradermal 61,65 and intranodal injections 66,67,68 . Notably, a recent report showed that repeated intranodal immunizations with naked, unmodified mRNA encoding tumour-associated neoantigens generated robust T cell responses and increased progression-free survival 68 (discussed further in Box 2).
Physical delivery methods in vivo. To increase the efficiency of mRNA uptake in vivo, physical methods have occasionally been used to penetrate the cell membrane. An early report showed that mRNA complexed with gold particles could be expressed in tissues using a gene gun, a microprojectile method 69 . The gene gun was shown to be an efficient RNA delivery and vaccination method in mouse models 70,71,72,73 , but no efficacy data in large animals or humans are available. In vivo electroporation has also been used to increase uptake of therapeutic RNA 74,75,76 ; however, in one study, electroporation increased the immunogenicity of only a self-amplifying RNA and not a non-replicating mRNA-based vaccine 74 . Physical methods can be limited by increased cell death and restricted access to target cells or tissues. Recently, the field has instead favoured the use of lipid or polymer-based nanoparticles as potent and versatile delivery vehicles.
Protamine. The cationic peptide protamine has been shown to protect mRNA from degradation by serum RNases 77 ; however, protamine-complexed mRNA alone demonstrated limited protein expression and efficacy in a cancer vaccine model, possibly owing to an overly tight association between protamine and mRNA 36,78 . This issue was resolved by developing the RNActive vaccine platform, in which protamine-formulated RNA serves only as an immune activator and not as an expression vector 52 .
Cationic lipid and polymer-based delivery. Highly efficient mRNA transfection reagents based on cationic lipids or polymers, such as TransIT-mRNA (Mirus Bio LLC) or Lipofectamine (Invitrogen), are commercially available and work well in many primary cells and cancer cell lines 9,13 , but they often show limited in vivo efficacy or a high level of toxicity (N.P. and D.W., unpublished observations). Great progress has been made in developing similarly designed complexing reagents for safe and effective in vivo use, and these are discussed in detail in several recent reviews 10,11,79,80 . Cationic lipids and polymers, including dendrimers, have become widely used tools for mRNA administration in the past few years. The mRNA field has clearly benefited from the substantial investment in in vivo small interfering RNA (siRNA) administration, where these delivery vehicles have been used for over a decade. Lipid nanoparticles (LNPs) have become one of the most appealing and commonly used mRNA delivery tools. LNPs often consist of four components: an ionizable cationic lipid, which promotes self-assembly into virus-sized (~100 nm) particles and allows endosomal release of mRNA to the cytoplasm; lipid-linked polyethylene glycol (PEG), which increases the half-life of formulations; cholesterol, a stabilizing agent; and naturally occurring phospholipids, which support lipid bilayer structure. Numerous studies have demonstrated efficient in vivo siRNA delivery by LNPs (reviewed in Ref. 81), but it has only recently been shown that LNPs are potent tools for in vivo delivery of self-amplifying RNA 19 and conventional, non-replicating mRNA 21 . Systemically delivered mRNA–LNP complexes mainly target the liver owing to binding of apolipoprotein E and subsequent receptor-mediated uptake by hepatocytes 82 , and intradermal, intramuscular and subcutaneous administration have been shown to produce prolonged protein expression at the site of the injection 21,22 . The mechanisms of mRNA escape into the cytoplasm are incompletely understood, not only for artificial liposomes but also for naturally occurring exosomes 83 . Further research into this area will likely be of great benefit to the field of therapeutic RNA delivery.
The magnitude and duration of in vivo protein production from mRNA–LNP vaccines can be controlled in part by varying the route of administration. Intramuscular and intradermal delivery of mRNA–LNPs has been shown to result in more persistent protein expression than systemic delivery routes: in one experiment, the half-life of mRNA-encoded firefly luciferase was roughly threefold longer after intradermal injection than after intravenous delivery 21 . These kinetics of mRNA–LNP expression may be favourable for inducing immune responses. A recent study demonstrated that sustained antigen availability during vaccination was a driver of high antibody titres and germinal centre (GC) B cell and T follicular helper (TFH) cell responses 84 . This process was potentially a contributing factor to the potency of recently described nucleoside-modified mRNA–LNP vaccines delivered by the intramuscular and intradermal routes 20,22,85 . Indeed, TFH cells have been identified as a critical population of immune cells that vaccines must activate in order to generate potent and long-lived neutralizing antibody responses, particularly against viruses that evade humoral immunity 86 . The dynamics of the GC reaction and the differentiation of TFH cells are incompletely understood, and progress in these areas would undoubtedly be fruitful for future vaccine design (Box 3).
Box 2: Personalized neoepitope cancer vaccines
Sahin and colleagues have pioneered the use of individualized neoepitope mRNA cancer vaccines 121 . They use high-throughput sequencing to identify every unique somatic mutation of an individual patient’s tumour sample, termed the mutanome. This enables the rational design of neoepitope cancer vaccines in a patient-specific manner, and has the advantage of targeting non-self antigen specificities that should not be eliminated by central tolerance mechanisms. Proof of concept has been recently provided: Kreiter and colleagues found that a substantial portion of non-synonymous cancer mutations were immunogenic when delivered by mRNA and were mainly recognized by CD4 + T cells 176 . On the basis of these data, they generated a computational method to predict major histocompatibility complex (MHC) class II-restricted neoepitopes that can be used as vaccine immunogens. mRNA vaccines encoding such neoepitopes have controlled tumour growth in B16-F10 melanoma and CT26 colon cancer mouse models. In a recent clinical trial, Sahin and colleagues developed personalized neoepitope-based mRNA vaccines for 13 patients with metastatic melanoma, a cancer known for its high frequency of somatic mutations and thus neoepitopes. They immunized against ten neoepitopes per individual by injecting naked mRNA intranodally. CD4 + T cell responses were detected against the majority of the neoepitopes, and a low frequency of metastatic disease was observed after several months of follow-up 68 . Interestingly, similar results were also obtained in a study of analogous design that used synthetic peptides as immunogens rather than mRNA 177 . Together, these recent trials suggest the potential utility of the personalized vaccine methodology.
Box 3: The germinal centre and T follicular helper cells
The vast majority of potent antimicrobial vaccines elicit long-lived, protective antibody responses against the target pathogen. High-affinity antibodies are produced in specialized microanatomical sites within the B cell follicles of secondary lymphoid organs called germinal centres (GCs). B cell proliferation, somatic hypermutation and selection for high-affinity mutants occur in the GCs, and efficient T cell help is required for these processes 178 . Characterization of the relationship between GC B and T cells has been actively studied in recent years. The follicular homing receptor CXC-chemokine receptor 5 (CXCR5) was identified on GC B and T cells in the 1990s 179,180 , but the concept of a specific lineage of T follicular helper (TFH) cells was not proposed until 2000 (Refs 181, 182). The existence of the TFH lineage was confirmed in 2009 when the transcription factor specific for TFH cells, B cell lymphoma 6 protein (BCL-6), was identified 183,184,185 . TFH cells represent a specialized subset of CD4 + T cells that produce critical signals for B cell survival, proliferation and differentiation in addition to signals for isotype switching of antibodies and for the introduction of diversifying mutations into the immunoglobulin genes. The major cytokines produced by TFH cells are interleukin-4 (IL-4) and IL-21, which play a key role in driving the GC reaction. Other important markers and functional ligands expressed by TFH cells include CD40 ligand (CD40L), Src homology domain 2 (SH2) domain-containing protein 1A (SH2D1A), programmed cell death protein 1 (PD1) and inducible T cell co-stimulator (ICOS) 186 . The characterization of rare, broadly neutralizing antibodies to HIV-1 has revealed that unusually high rates of somatic hypermutation are a hallmark of protective antibody responses against HIV-1 (Ref. 187). As TFH cells play a key role in driving this process in GC reactions, the development of new adjuvants or vaccine platforms that can potently activate this cell type is urgently needed.
mRNA vaccines against infectious diseases
Development of prophylactic or therapeutic vaccines against infectious pathogens is the most efficient means to contain and prevent epidemics. However, conventional vaccine approaches have largely failed to produce effective vaccines against challenging viruses that cause chronic or repeated infections, such as HIV-1, herpes simplex virus and respiratory syncytial virus (RSV). Additionally, the slow pace of commercial vaccine development and approval is inadequate to respond to the rapid emergence of acute viral diseases, as illustrated by the 2014–2016 outbreaks of the Ebola and Zika viruses. Therefore, the development of more potent and versatile vaccine platforms is crucial.
Preclinical studies have created hope that mRNA vaccines will fulfil many aspects of an ideal clinical vaccine: they have shown a favourable safety profile in animals, are versatile and rapid to design for emerging infectious diseases, and are amenable to scalable good manufacturing practice (GMP) production (already under way by several companies). Unlike protein immunization, several formats of mRNA vaccines induce strong CD8 + T cell responses, likely owing to the efficient presentation of endogenously produced antigens on MHC class I molecules, in addition to potent CD4 + T cell responses 56,87,88 . Additionally, unlike DNA immunization, mRNA vaccines have shown the ability to generate potent neutralizing antibody responses in animals with only one or two low-dose immunizations 20,22,85 . As a result, mRNA vaccines have elicited protective immunity against a variety of infectious agents in animal models 19,20,22,56,89,90 and have therefore generated substantial optimism. However, recently published results from two clinical trials of mRNA vaccines for infectious diseases were somewhat modest, leading to more cautious expectations about the translation of preclinical success to the clinic 22,91 (discussed further below).
Two major types of RNA vaccine have been utilized against infectious pathogens: self-amplifying or replicon RNA vaccines and non-replicating mRNA vaccines. Non-replicating mRNA vaccines can be further distinguished by their delivery method: ex vivo loading of DCs or direct in vivo injection into a variety of anatomical sites. As discussed below, a rapidly increasing number of preclinical studies in these areas have been published recently, and several have entered human clinical trials (Table 2).
Self-amplifying mRNA vaccines
Most currently used self-amplifying mRNA (SAM) vaccines are based on an alphavirus genome 92 , where the genes encoding the RNA replication machinery are intact but the genes encoding the structural proteins are replaced with the antigen of interest. The full-length RNA is ~9 kb long and can be easily produced by IVT from a DNA template. The SAM platform enables a large amount of antigen production from an extremely small dose of vaccine owing to intracellular replication of the antigen-encoding RNA. An early study reported that immunization with 10 μg of naked SAM vaccine encoding RSV fusion (F), influenza virus haemagglutinin (HA) or louping ill virus pre-membrane and envelope (prM-E) proteins resulted in antibody responses and partial protection from lethal viral challenges in mice 93 . The development of RNA complexing agents brought remarkable improvement to the efficacy of SAM vaccines. As little as 100 ng of an RNA replicon vaccine encoding RSV F, complexed to LNP, resulted in potent T and B cell immune responses in mice, and 1 μg elicited protective immune responses against RSV infection in a cotton rat intranasal challenge system 19 . SAM vaccines encoding influenza virus antigens in LNPs or an oil-in-water cationic nanoemulsion induced potent immune responses in ferrets and conferred protection from homologous and heterologous viral challenge in mice 94,95,96 . Further studies demonstrated the immunogenicity of this vaccine platform against diverse viruses in multiple species, including human cytomegalovirus (CMV), hepatitis C virus and rabies virus in mice, HIV-1 in rabbits, and HIV-1 and human CMV in rhesus macaques 50,87,97 . Replicon RNA encoding influenza antigens, complexed with chitosan-containing LNPs or polyethylenimine (PEI), has elicited T and B cell immune responses in mice after subcutaneous delivery 98,99 . Chahal and colleagues developed a delivery platform consisting of a chemically modified, ionizable dendrimer complexed into LNPs 89 . Using this platform, they demonstrated that intramuscular delivery of RNA replicons encoding influenza virus, Ebola virus or Toxoplasma gondii antigens protected mice against lethal infection 89 . The same group recently demonstrated that vaccination with an RNA replicon encoding Zika virus prM-E formulated in the same manner elicited antigen-specific antibody and CD8 + T cell responses in mice 88 . Another recent study reported immunogenicity and moderate protective efficacy of SAM vaccines against bacterial pathogens, namely Streptococcus (groups A and B) spp., further demonstrating the versatility of this platform 100 .
One of the advantages of SAM vaccines is that they create their own adjuvants in the form of dsRNA structures, replication intermediates and other motifs that may contribute to their high potency. However, the intrinsic nature of these PAMPs may make it difficult to modulate the inflammatory profile or reactogenicity of SAM vaccines. Additionally, size constraints of the insert are greater for SAM vaccines than for mRNAs that do not encode replicon genes, and the immunogenicity of the replication proteins may theoretically limit repeated use.
Dendritic cell mRNA vaccines
As described above, ex vivo DC loading is a heavily pursued method to generate cell-mediated immunity against cancer. Development of infectious disease vaccines using this approach has been mainly limited to a therapeutic vaccine for HIV-1: HIV-1-infected individuals on highly active antiretroviral therapy were treated with autologous DCs electroporated with mRNA encoding various HIV-1 antigens, and cellular immune responses were evaluated 101,102,103,104,105,106 . This intervention proved to be safe and elicited antigen-specific CD4 + and CD8 + T cell responses, but no clinical benefit was observed. Another study in humans evaluated a CMV pp65 mRNA-loaded DC vaccination in healthy human volunteers and allogeneic stem cell recipients and reported induction or expansion of CMV-specific cellular immune responses 107 .
Direct injection of non-replicating mRNA vaccines
Directly injectable, non-replicating mRNA vaccines are an appealing vaccine format owing to their simple and economical administration, particularly in resource-limited settings. Although an early report demonstrated that immunization with liposome-complexed mRNA encoding influenza virus nucleoproteins elicited CTL responses in mice 108 , the first demonstration of protective immune responses by mRNA vaccines against infectious pathogens was published only a few years ago 18 . This seminal work demonstrated that intradermally administered uncomplexed mRNA encoding various influenza virus antigens combined with a protamine-complexed RNA adjuvant was immunogenic in multiple animal models and protected mice from lethal viral challenge.
Immunization with the protamine-based RNActive platform encoding rabies virus glycoprotein has also induced protective immunity against a lethal intracerebral virus challenge in mice and potent neutralizing antibody responses in pigs 56 . In a recently published seminal work, Alberer and colleagues evaluated the safety and immunogenicity of this vaccine in 101 healthy human volunteers 91 . Subjects received 80–640 μg of mRNA vaccine three times by needle-syringe or needle-free devices, either intradermally or intramuscularly. Seven days after vaccination, nearly all participants reported mild to moderate injection site reactions, and 78% experienced a systemic reaction (for example, fever, headache and chills). There was one serious adverse event that was possibly related to the vaccine: a transient and moderate case of Bell palsy. Surprisingly, the needle-syringe injections did not generate detectable neutralizing antibodies in 98% of recipients. By contrast, needle-free delivery induced variable levels of neutralizing antibodies, the majority of which peaked above the expected protective threshold but then largely waned after 1 year in subjects who were followed up long term. Elucidating the basis of the disparate immunogenicity between the animals and humans who received this vaccine and between the two routes of delivery will be informative for future vaccine design using this platform.
Other infectious disease vaccines have successfully utilized lipid- or polymer-based delivery systems. Cationic 1,2-dioleoyloxy-3-trimethylammoniumpropane (DOTAP) and dioleoylphosphatidylethanolamine (DOPE) lipid-complexed mRNA encoding HIV-1 gag generated antigen-specific CD4 + and CD8 + T cell responses after subcutaneous delivery in mice 109 . Two other studies demonstrated that PEI-complexed mRNAs could be efficiently delivered to mice to induce HIV-1-specific immune responses: subcutaneously delivered mRNA encoding HIV-1 gag elicited CD4 + and CD8 + T cell responses, and intranasally administered mRNA encoding the HIV-1 envelope gp120 subunit crossed the nasal epithelium and generated antigen-specific immune responses in the nasal cavity 110,111 . Kranz and colleagues also performed intravenous immunizations in mice using lipid-complexed mRNA encoding influenza virus HA and showed evidence of T cell activation after a single dose 59 .
Nucleoside-modified mRNA vaccines represent a new and highly efficacious category of mRNA vaccines. Owing to the novelty of this immunization platform, our knowledge of efficacy is limited to the results of four recent publications that demonstrated the potency of such vaccines in small and large animals. The first published report demonstrated that a single intradermal injection of LNP-formulated mRNA encoding Zika virus prM-E, modified with 1-methylpseudouridine and FPLC purification, elicited protective immune responses in mice and rhesus macaques with the use of as little as 50 μg (0.02 mg kg −1 ) of vaccine in macaques 20 . A subsequent study by a different group tested a similarly designed vaccine against Zika virus in mice and found that a single intramuscular immunization elicited moderate immune responses, and a booster vaccination resulted in potent and protective immune responses 85 . This vaccine also incorporated the modified nucleoside 1-methylpseudouridine, but FPLC purification or other methods of removing dsRNA contaminants were not reported. Notably, this report showed that antibody-dependent enhancement of secondary infection with a heterologous flavivirus, a major concern for dengue and Zika virus vaccines, could be diminished by removing a cross-reactive epitope in the E protein. A recent follow-up study evaluated the same vaccine in a model of maternal vaccination and fetal infection 112 . Two immunizations reduced Zika virus infection in fetal mice by several orders of magnitude and completely rescued a defect in fetal viability.
Another recent report evaluated the immunogenicity of LNP-complexed, nucleoside-modified, non-FPLC-purified mRNA vaccines against influenza HA 10 neuraminidase 8 (H10N8) and H7N9 influenza viruses in mice, ferrets, non-human primates and, for the first time, humans 22 . A single intradermal or intramuscular immunization with low doses (0.4–10 μg) of LNP-complexed mRNA encoding influenza virus HA elicited protective immune responses against homologous influenza virus challenge in mice. Similar results were obtained in ferrets and cynomolgus monkeys after immunization with one or two doses of 50–400 μg of a vaccine containing LNP-complexed mRNA encoding HA, corroborating that the potency of mRNA–LNP vaccines translates to larger animals, including non-human primates.
On the basis of encouraging preclinical data, two phase I clinical trials have recently been initiated to evaluate the immunogenicity and safety of nucleoside-modified mRNA–LNP vaccines in humans for the first time. The mRNA vaccine encoding H10N8 HA is currently undergoing clinical testing (NCT03076385), and interim findings for 23 vaccinated individuals have been reported 22 . Participants received a small amount (100 μg) of vaccine intramuscularly, and immunogenicity was measured 43 days after vaccination. The vaccine proved to be immunogenic in all subjects, as measured by haemagglutination inhibition and microneutralization antibody assays. Promisingly, antibody titres were above the expected protective threshold, but they were moderately lower than in the animal models. Similarly to the study by Alberer et al. 91 , most vaccinated subjects reported mild to moderate reactogenicity (injection site pain, myalgia, headache, fatigue and chills), and three subjects reported severe injection site reactions or a systemic common cold-like response. This level of reactogenicity appears to be similar to that of more traditional vaccine formats 113,114 . Finally, the Zika virus vaccine described by Richner et al. 85,112 is also entering clinical evaluation in a combined phase I/II trial (NCT03014089). Future studies that apply nucleoside-modified mRNA–LNP vaccines against a greater diversity of antigens will reveal the extent to which this strategy is broadly applicable to infectious disease vaccines.
mRNA cancer vaccines
mRNA-based cancer vaccines have been recently and extensively reviewed 115,116,117,118,119 . Below, the most recent advances and directions are highlighted. Cancer vaccines and other immunotherapies represent promising alternative strategies to treat malignancies. Cancer vaccines can be designed to target tumour-associated antigens that are preferentially expressed in cancerous cells, for example, growth-associated factors, or antigens that are unique to malignant cells owing to somatic mutation 120 . These neoantigens, or the neoepitopes within them, have been deployed as mRNA vaccine targets in humans 121 (Box 2). Most cancer vaccines are therapeutic, rather than prophylactic, and seek to stimulate cell-mediated responses, such as those from CTLs, that are capable of clearing or reducing tumour burden 122 . The first proof-of-concept studies that not only proposed the idea of RNA cancer vaccines but also provided evidence of the feasibility of this approach were published more than two decades ago 123,124 . Since then, numerous preclinical and clinical studies have demonstrated the viability of mRNA vaccines to combat cancer (Table 3).
DC mRNA cancer vaccines
As DCs are central players in initiating antigen-specific immune responses, it seemed logical to utilize them for cancer immunotherapy. The first demonstration that DCs electroporated with mRNA could elicit potent immune responses against tumour antigens was reported by Boczkowski and colleagues in 1996 (Ref. 124). In this study, DCs pulsed with ovalbumin (OVA)-encoding mRNA or tumour-derived RNAs elicited a tumour-reducing immune response in OVA-expressing and other melanoma models in mice. A variety of immune regulatory proteins have been identified in the form of mRNA-encoded adjuvants that can increase the potency of DC cancer vaccines. Several studies demonstrated that electroporation of DCs with mRNAs encoding co-stimulatory molecules such as CD83, tumour necrosis factor receptor superfamily member 4 (TNFRSF4; also known as OX40) and 4-1BB ligand (4-1BBL) resulted in a substantial increase in the immune stimulatory activity of DCs 125,126,127,128 . DC functions can also be modulated through the use of mRNA-encoded pro-inflammatory cytokines, such as IL-12, or trafficking-associated molecules 129,130,131 . As introduced above, TriMix is a cocktail of mRNA-encoded adjuvants (CD70, CD40L and constitutively active TLR4) that can be electroporated in combination with antigen-encoding mRNA or mRNAs 132 . This formulation proved efficacious in multiple preclinical studies by increasing DC activation and shifting the CD4 + T cell phenotype from T regulatory cells to T helper 1 (TH1)-like cells 132,133,134,135,136 . Notably, the immunization of patients with stage III or stage IV melanoma using DCs loaded with mRNA encoding melanoma-associated antigens and TriMix adjuvant resulted in tumour regression in 27% of treated individuals 137 . Multiple clinical trials have now been conducted using DC vaccines targeting various cancer types, such as metastatic prostate cancer, metastatic lung cancer, renal cell carcinoma, brain cancers, melanoma, acute myeloid leukaemia, pancreatic cancer and others 138,139 (reviewed in Refs 51,58).
A new line of research combines mRNA electroporation of DCs with traditional chemotherapy agents or immune checkpoint inhibitors. In one trial, patients with stage III or IV melanoma were treated with ipilimumab, a monoclonal antibody against CTL antigen 4 (CTLA4), and DCs loaded with mRNA encoding melanoma-associated antigens plus TriMix. This intervention resulted in durable tumour reduction in a proportion of individuals with recurrent or refractory melanoma 140 .
Direct injection of mRNA cancer vaccines
The route of administration and delivery format of mRNA vaccines can greatly influence outcomes. A variety of mRNA cancer vaccine formats have been developed using common delivery routes (intradermal, intramuscular, subcutaneous or intranasal) and some unconventional routes of vaccination (intranodal, intravenous, intrasplenic or intratumoural).
Intranodal administration of naked mRNA is an unconventional but efficient means of vaccine delivery. Direct mRNA injection into secondary lymphoid tissue offers the advantage of targeted antigen delivery to antigen-presenting cells at the site of T cell activation, obviating the need for DC migration. Several studies have demonstrated that intranodally injected naked mRNA can be selectively taken up by DCs and can elicit potent prophylactic or therapeutic antitumour T cell responses 62,66 ; an early study also demonstrated similar findings with intrasplenic delivery 141 . Coadministration of the DC-activating protein FMS-related tyrosine kinase 3 ligand (FLT3L) was shown in some cases to further improve immune responses to intranodal mRNA vaccination 142,143 . Incorporation of the TriMix adjuvant into intranodal injections of mice with mRNAs encoding tumour-associated antigens resulted in potent antigen-specific CTL responses and tumour control in multiple tumour models 133 . A more recent study demonstrated that intranodal injection of mRNA encoding the E7 protein of human papillomavirus (HPV) 16 with TriMix increased the number of tumour-infiltrating CD8 + T cells and inhibited the growth of an E7-expressing tumour model in mice 67 .
The success of preclinical studies has led to the initiation of clinical trials using intranodally injected naked mRNA encoding tumour-associated antigens into patients with advanced melanoma (NCT01684241) and patients with hepatocellular carcinoma (EudraCT: 2012-005572-34). In one published trial, patients with metastatic melanoma were treated with intranodally administered DCs electroporated with mRNA encoding the melanoma-associated antigens tyrosinase or gp100 and TriMix, which induced limited antitumour responses 144 .
Intranasal vaccine administration is a needle-free, noninvasive manner of delivery that enables rapid antigen uptake by DCs. Intranasally delivered mRNA complexed with Stemfect (Stemgent) LNPs resulted in delayed tumour onset and increased survival in prophylactic and therapeutic mouse tumour models using the OVA-expressing E.G7-OVA T lymphoblastic cell line 145 .
Intratumoural mRNA vaccination is a useful approach that offers the advantage of rapid and specific activation of tumour-resident T cells. Often, these vaccines do not introduce mRNAs encoding tumour-associated antigens but simply aim to activate tumour-specific immunity in situ using immune stimulatory molecules. An early study demonstrated that naked mRNA or protamine-stabilized mRNA encoding a non-tumour related gene (GLB1) impaired tumour growth and provided protection in a glioblastoma mouse model, taking advantage of the intrinsic immunogenic properties of mRNA 146 . A more recent study showed that intratumoural delivery of mRNA encoding an engineered cytokine based on interferon-β (IFNβ) fused to a transforming growth factor-β (TGFβ) antagonist increased the cytolytic capacity of CD8 + T cells and modestly delayed tumour growth in OVA-expressing lymphoma or lung carcinoma mouse models 147 . It has also been shown that intratumoural administration of TriMix mRNA that does not encode tumour-associated antigens results in activation of CD8α + DCs and tumour-specific T cells, leading to delayed tumour growth in various mouse models 148 .
Systemic administration of mRNA vaccines is not common owing to concerns about aggregation with serum proteins and rapid extracellular mRNA degradation; thus, formulating mRNAs into carrier molecules is essential. As discussed above, numerous delivery formulations have been developed to facilitate mRNA uptake, increase protein translation and protect mRNA from RNases 10,11,79,80 . Another important issue is the biodistribution of mRNA vaccines after systemic delivery. Certain cationic LNP-based complexing agents delivered intravenously traffic mainly to the liver 21 , which may not be ideal for DC activation. An effective strategy for DC targeting of mRNA vaccines after systemic delivery has recently been described 59 . An mRNA–lipoplex (mRNA–liposome complex) delivery platform was generated using cationic lipids and neutral helper lipids formulated with mRNA, and it was discovered that the lipid-to-mRNA ratio, and thus the net charge of the particles, has a profound impact on the biodistribution of the vaccine. While a positively charged lipid particle primarily targeted the lung, a negatively charged particle targeted DCs in secondary lymphoid tissues and bone marrow. The negatively charged particle induced potent immune responses against tumour-specific antigens that were associated with impressive tumour reduction in various mouse models 59 . As no toxic effects were observed in mice or non-human primates, clinical trials using this approach to treat patients with advanced melanoma or triple-negative breast cancer have been initiated (NCT02410733 and NCT02316457).
A variety of antigen-presenting cells reside in the skin 149 , making it an ideal site for immunogen delivery during vaccination (Fig. 3). Thus, the intradermal route of delivery has been widely used for mRNA cancer vaccines. An early seminal study demonstrated that intradermal administration of total tumour RNA delayed tumour growth in a fibrosarcoma mouse model 65 . Intradermal injection of mRNA encoding tumour antigens in the protamine-based RNActive platform proved efficacious in various mouse models of cancer 36 and in multiple prophylactic and therapeutic clinical settings (Table 3). One such study demonstrated that mRNAs encoding survivin and various melanoma tumour antigens resulted in increased numbers of antigen-specific T cells in a subset of patients with melanoma 150 . In humans with castration-resistant prostate cancer, an RNActive vaccine expressing multiple prostate cancer-associated proteins elicited antigen-specific T cell responses in the majority of recipients 151 . Lipid-based carriers have also contributed to the efficacy of intradermally delivered mRNA cancer vaccines. The delivery of OVA-encoding mRNA in DOTAP and/or DOPE liposomes resulted in antigen-specific CTL activity and inhibited growth of OVA-expressing tumours in mice 152 . In the same study, coadministration of mRNA encoding granulocyte–macrophage colony-stimulating factor (GM-CSF) improved OVA-specific cytolytic responses. Another report showed that subcutaneous delivery of LNP-formulated mRNA encoding two melanoma-associated antigens delayed tumour growth in mice, and co-delivery of lipopolysaccharide (LPS) in LNPs increased both CTL and antitumour activity 153 . In general, mRNA cancer vaccines have proved immunogenic in humans, but further refinement of vaccination methods, as informed by basic immunological research, will likely be necessary to achieve greater clinical benefits.
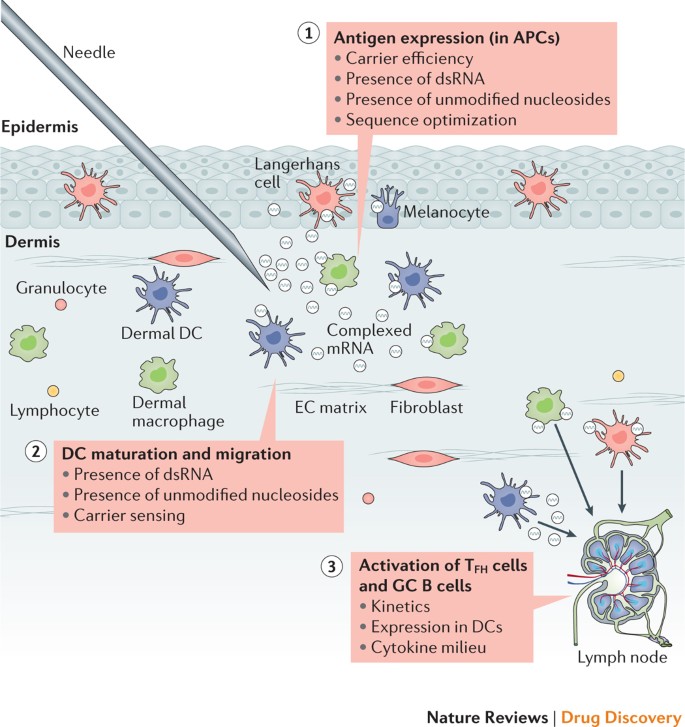
For an injected mRNA vaccine, major considerations for effectiveness include the following: the level of antigen expression in professional antigen-presenting cells (APCs), which is influenced by the efficiency of the carrier, by the presence of pathogen-associated molecular patterns (PAMPs) in the form of double-stranded RNA (dsRNA) or unmodified nucleosides and by the level of optimization of the RNA sequence (codon usage, G:C content, 5′ and 3′ untranslated regions (UTRs) and so on); dendritic cell (DC) maturation and migration to secondary lymphoid tissue, which is increased by PAMPs; and the ability of the vaccine to activate robust T follicular helper (TFH) cell and germinal centre (GC) B cell responses — an area that remains poorly understood. An intradermal injection is shown as an example. EC, extracellular.
The combination of mRNA vaccination with adjunctive therapies, such as traditional chemotherapy, radiotherapy and immune checkpoint inhibitors, has increased the beneficial outcome of vaccination in some preclinical studies 154,155 . For example, cisplatin treatment significantly increased the therapeutic effect of immunizing with mRNA encoding the HPV16 E7 oncoprotein and TriMix, leading to the complete rejection of female genital tract tumours in a mouse model 67 . Notably, it has also been suggested that treatment with antibodies against programmed cell death protein 1 (PD1) increased the efficacy of a neoepitope mRNA-based vaccine against metastatic melanoma in humans, but more data are required to explore this hypothesis 68 .
Therapeutic considerations and challenges
Good manufacturing practice production
mRNA is produced by in vitro reactions with recombinant enzymes, ribonucleotide triphosphates (NTPs) and a DNA template; thus, it is rapid and relatively simple to produce in comparison with traditional protein subunit and live or inactivated virus vaccine production platforms. Its reaction yield and simplicity make rapid mRNA production possible in a small GMP facility footprint. The manufacturing process is sequence-independent and is primarily dictated by the length of the RNA, the nucleotide and capping chemistry and the purification of the product; however, it is possible that certain sequence properties such as extreme length may present difficulties (D.W., unpublished observations). According to current experience, the process can be standardized to produce nearly any encoded protein immunogen, making it particularly suitable for rapid response to emerging infectious diseases.
All enzymes and reaction components required for the GMP production of mRNA can be obtained from commercial suppliers as synthesized chemicals or bacterially expressed, animal component-free reagents, thereby avoiding safety concerns surrounding the adventitious agents that plague cell-culture-based vaccine manufacture. All the components, such as plasmid DNA, phage polymerases, capping enzymes and NTPs, are readily available as GMP-grade traceable components; however, some of these are currently available at only limited scale or high cost. As mRNA therapeutics move towards commercialization and the scale of production increases, more economical options may become accessible for GMP source materials.
GMP production of mRNA begins with DNA template production followed by enzymatic IVT and follows the same multistep protocol that is used for research scale synthesis, with added controls to ensure the safety and potency of the product 16 . Depending on the specific mRNA construct and chemistry, the protocol may be modified slightly from what is described here to accommodate modified nucleosides, capping strategies or template removal. To initiate the production process, template plasmid DNA produced in Escherichia coli is linearized using a restriction enzyme to allow synthesis of runoff transcripts with a poly(A) tract at the 3′ end. Next, the mRNA is synthesized from NTPs by a DNA-dependent RNA polymerase from bacteriophage (such as T7, SP6, or T3). The template DNA is then degraded by incubation with DNase. Finally, the mRNA is enzymatically or chemically capped to enable efficient translation in vivo. mRNA synthesis is highly productive, yielding in excess of 2 g l −1 of full-length mRNA in multi-gram scale reactions under optimized conditions.
Once the mRNA is synthesized, it is processed though several purification steps to remove reaction components, including enzymes, free nucleotides, residual DNA and truncated RNA fragments. While LiCl precipitation is routinely used for laboratory-scale preparation, purification at the clinical scale utilizes derivatized microbeads in batch or column formats, which are easier to utilize at large scale 156,157 . For some mRNA platforms, removal of dsRNA and other contaminants is critical for the potency of the final product, as it is a potent inducer of interferon-dependent translation inhibition. This has been accomplished by reverse-phase FPLC at the laboratory scale 158 , and scalable aqueous purification approaches are being investigated. After mRNA is purified, it is exchanged into a final storage buffer and sterile-filtered for subsequent filling into vials for clinical use. RNA is susceptible to degradation by both enzymatic and chemical pathways 157 . Formulation buffers are tested to ensure that they are free of contaminating RNases and may contain buffer components, such as antioxidants and chelators, which minimize the effects of reactive oxygen species and divalent metal ions that lead to mRNA instability 159 .
Pharmaceutical formulation of mRNAs is an active area of development. Although most products for early phase studies are stored frozen (−70 °C), efforts to develop formulations that are stable at higher temperatures more suitable for vaccine distribution are continuing. Published reports suggest that stable refrigerated or room temperature formulations can be made. The RNActive platform was reported to be active after lyophilization and storage at 5–25 °C for 3 years and at 40 °C for 6 months 91 . Another report demonstrated that freeze-dried naked mRNA is stable for at least 10 months under refrigerated conditions 160 . The stability of mRNA products might also be improved by packaging within nanoparticles or by co-formulation with RNase inhibitors 161 . For lipid-encapsulated mRNA, at least 6 months of stability has been observed (Arbutus Biopharma, personal communication), but longer-term storage of such mRNA–lipid complexes in an unfrozen form has not yet been reported.
Regulatory aspects
There is no specific guidance from the FDA or European Medicines Agency (EMA) for mRNA vaccine products. However, the increasing number of clinical trials conducted under EMA and FDA oversight indicate that regulators have accepted the approaches proposed by various organizations to demonstrate that products are safe and acceptable for testing in humans. Because mRNA falls into the broad vaccine category of genetic immunogens, many of the guiding principles that have been defined for DNA vaccines 162 and gene therapy vectors 163,164 can likely be applied to mRNA with some adaptations to reflect the unique features of mRNA. A detailed review of EMA regulations for RNA vaccines by Hinz and colleagues highlights the different regulatory paths stipulated for prophylactic infectious disease versus therapeutic applications 165 . Regardless of the specific classification within existing guidelines, some themes can be observed in what is stated in these guidance documents and in what has been reported for recently published clinical studies. In particular, the recent report of an mRNA vaccine against influenza virus highlights preclinical and clinical data demonstrating biodistribution and persistence in mice, disease protection in a relevant animal model (ferrets), and immunogenicity, local reactogenicity and toxicity in humans 22 . As mRNA products become more prominent in the vaccine field, it is likely that specific guidance will be developed that will delineate requirements to produce and evaluate new mRNA vaccines.
Safety
The requirement for safety in modern prophylactic vaccines is extremely stringent because the vaccines are administered to healthy individuals. Because the manufacturing process for mRNA does not require toxic chemicals or cell cultures that could be contaminated with adventitious viruses, mRNA production avoids the common risks associated with other vaccine platforms, including live virus, viral vectors, inactivated virus and subunit protein vaccines. Furthermore, the short manufacturing time for mRNA presents few opportunities to introduce contaminating microorganisms. In vaccinated people, the theoretical risks of infection or integration of the vector into host cell DNA are not a concern for mRNA. For the above reasons, mRNA vaccines have been considered a relatively safe vaccine format.
Several different mRNA vaccines have now been tested from phase I to IIb clinical studies and have been shown to be safe and reasonably well tolerated (Tables 2, 3). However, recent human trials have demonstrated moderate and in rare cases severe injection site or systemic reactions for different mRNA platforms 22,91 . Potential safety concerns that are likely to be evaluated in future preclinical and clinical studies include local and systemic inflammation, the biodistribution and persistence of expressed immunogen, stimulation of auto-reactive antibodies and potential toxic effects of any non-native nucleotides and delivery system components. A possible concern could be that some mRNA-based vaccine platforms 54,166 induce potent type I interferon responses, which have been associated not only with inflammation but also potentially with autoimmunity 167,168 . Thus, identification of individuals at an increased risk of autoimmune reactions before mRNA vaccination may allow reasonable precautions to be taken. Another potential safety issue could derive from the presence of extracellular RNA during mRNA vaccination. Extracellular naked RNA has been shown to increase the permeability of tightly packed endothelial cells and may thus contribute to oedema 169 . Another study showed that extracellular RNA promoted blood coagulation and pathological thrombus formation 170 . Safety will therefore need continued evaluation as different mRNA modalities and delivery systems are utilized for the first time in humans and are tested in larger patient populations.
Conclusions and future directions
Currently, mRNA vaccines are experiencing a burst in basic and clinical research. The past 2 years alone have witnessed the publication of dozens of preclinical and clinical reports showing the efficacy of these platforms. Whereas the majority of early work in mRNA vaccines focused on cancer applications, a number of recent reports have demonstrated the potency and versatility of mRNA to protect against a wide variety of infectious pathogens, including influenza virus, Ebola virus, Zika virus, Streptococcus spp. and T. gondii (Tables 1,2).
While preclinical studies have generated great optimism about the prospects and advantages of mRNA-based vaccines, two recent clinical reports have led to more tempered expectations 22,91 . In both trials, immunogenicity was more modest in humans than was expected based on animal models, a phenomenon also observed with DNA-based vaccines 171 , and the side effects were not trivial. We caution that these trials represent only two variations of mRNA vaccine platforms, and there may be substantial differences when the expression and immunostimulatory profiles of the vaccine are changed. Further research is needed to determine how different animal species respond to mRNA vaccine components and inflammatory signals and which pathways of immune signalling are most effective in humans.
Recent advances in understanding and reducing the innate immune sensing of mRNA have aided efforts not only in active vaccination but also in several applications of passive immunization or passive immunotherapy for infectious diseases and cancer (Box 4). Direct comparisons between mRNA expression platforms should clarify which systems are most appropriate for both passive and active immunization. Given the large number of promising mRNA platforms, further head-to-head comparisons would be of utmost value to the vaccine field because this would allow investigators to focus resources on those best suited for each application.
The fast pace of progress in mRNA vaccines would not have been possible without major recent advances in the areas of innate immune sensing of RNA and in vivo delivery methods. Extensive basic research into RNA and lipid and polymer biochemistry has made it possible to translate mRNA vaccines into clinical trials and has led to an astonishing level of investment in mRNA vaccine companies (Table 4). Moderna Therapeutics, founded in 2010, has raised almost US$2 billion in capital with a plan to commercialize mRNA-based vaccines and therapies 172,173 . The US Biomedical Advanced Research and Development Authority (BARDA) has committed support for Moderna’s clinical evaluation of a promising nucleoside-modified mRNA vaccine for Zika virus (NCT03014089). In Germany, CureVac AG has an expanding portfolio of therapeutic targets 174 , including both cancer and infectious diseases, and BioNTech is developing an innovative approach to personalized cancer medicine using mRNA vaccines 121 (Box 2). The translation of basic research into clinical testing is also made more expedient by the commercialization of custom GMP products by companies such as New England Biolabs and Aldevron 175 . Finally, the recent launch of the Coalition for Epidemic Preparedness Innovations (CEPI) provides great optimism for future responses to emerging viral epidemics. This multinational public and private partnership aims to raise $1 billion to develop platform-based vaccines, such as mRNA, to rapidly contain emerging outbreaks before they spread out of control.
The future of mRNA vaccines is therefore extremely bright, and the clinical data and resources provided by these companies and other institutions are likely to substantially build on and invigorate basic research into mRNA-based therapeutics.
Box 4: mRNA-based passive immunotherapy
Recombinant monoclonal antibodies are rapidly transforming the pharmaceutical market and have become one of the most successful therapeutic classes to treat autoimmune disorders, infectious diseases, osteoporosis, hypercholesterolemia and cancer 188,189,190,191,192 . However, the high cost of protein production and the need for frequent systemic administration pose a major limitation to widespread accessibility. Antibody-gene transfer technologies could potentially overcome these difficulties, as they administer nucleotide sequences encoding monoclonal antibodies to patients, enabling in vivo production of properly folded and modified protein therapeutics 193 . Multiple gene therapy vectors have been investigated (for example, viral vectors and plasmid DNA) that bear limitations such as pre-existing host immunity, acquired anti-vector immunity, high innate immunogenicity, difficulties with in vivo regulation of antibody production and toxic effects 193,194 . mRNA therapeutics combine safety with exquisite dose control and the potential for multiple administrations with no pre-existing or anti-vector immunity. Two early reports demonstrated that dendritic cells (DCs) electroporated with mRNAs encoding antibodies against immuno-inhibitory proteins secreted functional antibodies and improved immune responses in mice 195,196 . Three recent publications have described the use of injectable mRNA for in vivo production of therapeutic antibodies: Pardi and colleagues demonstrated that a single intravenous injection into mice with lipid nanoparticle (LNP)-encapsulated nucleoside-modified mRNAs encoding the heavy and light chains of the anti-HIV-1 neutralizing antibody VRC01 rapidly produced high levels of functional antibody in the serum and protected humanized mice from HIV-1 infection 197 ; Stadler and co-workers demonstrated that intravenous administration of low doses of TransIT (Mirus Bio LLC)-complexed, nucleoside-modified mRNAs encoding various anticancer bispecific antibodies resulted in the elimination of large tumours in mouse models 198 ; and Thran and colleagues 199 utilized an unmodified mRNA–LNP delivery system 12 to express three monoclonal antibodies at levels that protected from lethal challenges with rabies virus, botulinum toxin and a B cell lymphoma cell line. No toxic effects were observed in any of these studies. These observations suggest that mRNA offers a safe, simple and efficient alternative to therapeutic monoclonal antibody protein delivery, with potential application to any therapeutic protein.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source https://www.nature.com/articles/s41577-021-00583-2
Source https://www.nature.com/articles/nrd.2017.243
Source